Patients with anomalous coronary arteries arising from the opposite sinus of Valsalva (ACAOS), the left coronary artery (LCA) arising from the right sinus or the right coronary artery (RCA) from the left sinus with an interarterial course, may present from complete absence of symptoms to sudden cardiac death. Although there are guidelines on indications for surgery, controversy remains.
MethodsA retrospective review was performed of all adult patients diagnosed with ACAOS in our hospital between 2007 and 2016. Demographic, clinical, perioperative and follow-up data were collected from clinical records and summarized. A review of the published literature was performed with special emphasis on clinical presentation, surgical indications and results.
ResultsSeven symptomatic patients underwent surgery (mean age 57.1±8.9 years, two male, five female); they recovered without complications and to date have had no recurrence of myocardial ischemia. One asymptomatic patient with an anomalous RCA has been medically followed without evidence of myocardial ischemia. A 75-year-old woman, diagnosed in 2008 with an anomalous LCA, was not referred for surgery and died suddenly six months after diagnosis.
ConclusionsSurgery for coronary abnormalities is performed with low risk and all published series report full operative survival. The indications for surgery are well established for patients with interarterial anomalous LCA and symptomatic patients with interarterial anomalous RCA. However, there is some uncertainty concerning asymptomatic patients, particularly those with an anomalous interarterial RCA, for whom we propose a more assertive approach, if young or engaged in strenuous activities.
Doentes com origem anómala de uma artéria coronária no seio coronário oposto (OAAC – artéria coronária esquerda, ACE, proveniente do seio direito ou artéria coronária direita, ACD, originária do seio esquerdo) com um percurso interarterial podem apresentar desde ausência de sintomas até morte súbita. Apesar da existência de guidelines para orientação cirúrgica, controvérsias persistem.
MétodosFoi feito um estudo retrospetivo de todos os doentes adultos diagnosticados com OAAC no Hospital de S. João, entre 2007 e 2016. Foram recolhidos dados demográficos, clínicos, perioperatórios e do seguimento. Uma revisão da literatura foi feita, enfatizou a apresentação clínica, as indicações e os resultados cirúrgicos.
ResultadosSete doentes sintomáticos foram submetidos a cirurgia (a média de idade foi de 57,1 ± 8,9 anos; dois homens, cinco mulheres), recuperaram sem complicações e sem recorrência de isquemia do miocárdio. Uma doente jovem assintomática, com uma ACD anómala, mantém-se em seguimento, sem intervenção e sem evidência de isquemia. O primeiro doente da série, sexo feminino, 75 anos, foi diagnosticada em 2008 com ACE anómala, não foi proposta para cirurgia e morreu subitamente seis meses após o diagnóstico.
ConclusõesA cirurgia das anomalias coronárias é feita com risco baixo e sem mortalidade operatória em todas as séries publicadas. As indicações para cirurgia estão bem estabelecidas para doentes com ACE anómala e trajeto interarterial e em doentes sintomáticos com ACD anómala. Contudo, permanecem incertezas em doentes assintomáticos, particularmente naqueles que apresentam uma ACD anómala com trajeto interarterial, para os quais propomos uma abordagem mais interventiva, se jovens ou sujeitos a atividades vigorosas.
Coronary artery anomalies (CAAs) are a diverse group of congenital disorders with highly variable manifestations and pathophysiological mechanisms.1
The wide spectrum of CAAs has been comprehensively organized by Paolo Angelini,2 who divided them into anomalies of origin and course; anomalies of intrinsic coronary arterial anatomy; anomalies of coronary termination; and anomalous anastomotic vessels. The terms ‘anomalous’ or ‘abnormal’ are generally used to define any variant form of coronary arterial anatomy; variant forms are observed in less than 1% of the general population.3 Isolated CAAs have been described in 0.5% of patients undergoing coronary angiography,4,5 0.3% of individuals at autopsy6 and 0.17% in a prospective echocardiographic series.7 An anomalous coronary artery arising from the opposite sinus of Valsalva (ACAOS) is the subgroup of coronary anomalies with the most potential for clinical repercussions in adults, especially sudden cardiac death (SCD).2
A systematic review of the diagnostic databases of the cardiology and cardiothoracic surgery departments of Centro Hospitalar S. João retrieved a total of nine adult patients with ACAOS between 2007 and 2016. Although the first paper published in Portugal reporting the surgical repair of an ACAOS dates from 2006,8 this is the first surgical series so far reported in this country.
MethodsThis retrospective study was performed by reviewing the clinical records of all patients at our institution, including coronary angiograms, computed tomography (CT) coronary angiograms and follow-up consultations. Patients with CAAs other than ACAOS were excluded. Individual reports were categorized by demographics, clinical presentation, method of diagnosis, type and course of coronary anomaly, type of surgery, time of cardiopulmonary bypass and aortic clamping, length of hospital stay and postoperative complications (Tables 1 and 2).
Patient characteristics, anatomical diagnoses and surgical techniques.
| Case | Age | Symptoms | Diagnosis | Anatomy | Course | Associated lesions | Operation |
|---|---|---|---|---|---|---|---|
| 1 | 75 | ACS | CA | LCA from right sinus | Intramural | None | No surgery |
| 2 | 70 | Angina | CA | RCA from left sinus | Intramural | None | Bypass RITA to RCA |
| 3 | 56 | ACS | CA | RCA from left sinus | Intramyocardial | None | Anastomosis RCA-aorta |
| 4 | 57 | Inferior STEMI | CA | LCA from right sinus | Intramural | Occlusion of posterior RCA | LCA fenestration – neo-ostium |
| 5 | 67 | CHF (NYHA III); no angina | CA | RCA from left sinus | Intramural | Rheumatic triple valve disease | Unroofing |
| 6 | 54 | NSTEMI | CA | RCA from left sinus | Short, intramural | None | RCA reimplantation |
| 7 | 47 | Cardiac arrest | CA | LCA from right sinus | Intramural | None | Unroofing |
| 8 | 22 | Atypical chest pain | CT | RCA from left sinus | Intramural | None | No surgery |
| 9 | 46 | Chest pain | CT | LCA from right sinus | Intramural | None | LCA fenestration – neo-ostium |
ACS: acute coronary syndrome; Age: age at diagnosis in years; CA: coronary angiography; CHF: congestive heart failure; CT: computed tomography coronary angiography; LCA: left coronary artery; NSTEMI: non-ST-elevation myocardial infarction; NYHA: New York Association functional class; RCA: right coronary artery; RITA: right internal thoracic artery; STEMI: ST-elevation myocardial infarction.
Operative and postoperative details.
| Case | Surgery | LoS | Clinical status | Postoperative exams | FUP | ||
|---|---|---|---|---|---|---|---|
| Echo | Stress test | Imaging | |||||
| 1 | No | - | Sudden death | - | - | - | |
| 2 | Off-pump RITA-RCA | 11 | Asymptomatic | Normal | Negative | RCA-RITA graft patent; RCA proximal 90% stenosis | 113 |
| 3 | Anastomosis RCA-aorta | 5 | Asymptomatic | Normal | Negative | Anastomosis open on CT | 58 |
| 4 | LCA neo-ostium | 7 | Asymptomatic | Inferior akinesia | Mild inferior defect | - | 44 |
| 5 | TVS; RCA unroofing | 21 | Dead (cancer) | Normal | - | - | 22 |
| 6 | RCA reimplantation | 5 | Asymptomatic | Normal | Negative | Normal CA | 19 |
| 7 | LCA unroofing | 5 | Asymptomatic | Normal | Negative | Anastomosis open on CT | 17 |
| 8 | - | - | Asymptomatic | . | - | - | |
| 9 | LCA neo-ostium | 7 | Asymptomatic | Normal | Negative | - | 7 |
CA: coronary angiography; CT: computed tomography coronary angiography; Echo: echocardiography; FUP: follow-up time in months; LCA: left coronary artery; LoS: length of stay in days; RCA: right coronary artery; RITA: right internal thoracic artery; TVS: triple valve surgery.
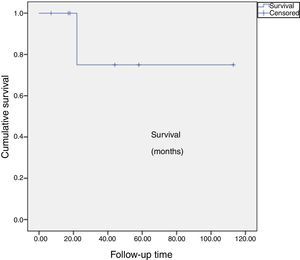
Outcomes were assessed clinically for angina, myocardial infarction, death and cause, and results of postoperative examinations (Table 2). Survival, follow-up time and freedom from cardiac events were calculated (Figure 1).
Statistical analysis was performed with IBM SPSS Statistics 24.0.9 Sample statistics are presented as mean and standard deviation or median and interquartile range, according to distribution. Survival data were computed by the Kaplan-Meier method.
A literature review of observational studies was performed on PubMed, using the search term “coronary anomalies” in the Title/Abstract, limited to papers published between January 2000 and June 2016. Exclusion criteria were the reporting of coronary anomalies other than origin from the opposite sinus and language other than English, Portuguese or Spanish. Four additional papers published before 2000 were included by cross-reference. One additional paper, published in March 2017, was included after editorial review. Significant publications with special emphasis on clinical presentation, diagnosis, surgical indications and operative results were read and major contributions referenced in the discussion.
ResultsNine adult patients comprised this study group, as no children were identified in the databases with this diagnosis. Four of them had the left coronary artery (LCA) originating from the right sinus of Valsalva, and in the other five the right coronary artery (RCA) originated from the left sinus.
Seven of these patients were proposed and accepted for surgery and operated with success by the same surgeon (JC). Three patients had an LCA from the right sinus and the other four had an RCA from the left sinus. In one, the coronary course was extramural and intramyocardial, and in the other six it was intramural. Mean age at surgery was 57.1±8.9 years; two patients were male and five female. Four had hypertension and dyslipidemia and none had diabetes. A 75-year-old woman, diagnosed in 2008 with an LCA from the right sinus, symptoms of heart failure and severe respiratory insufficiency, was not referred for surgery and died suddenly six months after diagnosis. An asymptomatic female patient, 22 years old and a firefighter, performed several stress tests without evidence of ischemia and has been followed medically, but had to quit her job.
Surgical revascularization techniques commonly used to address ACAOS include:
- -
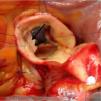
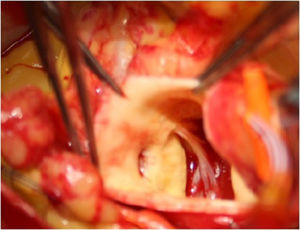
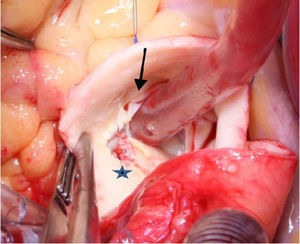
Ostial reimplantation, involving detachment of the coronary button and attachment in the correct sinus (Figure 2), which was performed in case 6.
- -
In case 3, the course of the coronary was extramural and intramyocardial, hence reimplantation would be our first choice. However, this technique could have jeopardized a large right infundibular branch and a lateral anastomosis was created in the right sinus, thereby creating a coronary artery with two ostia10;
- -
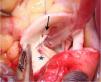
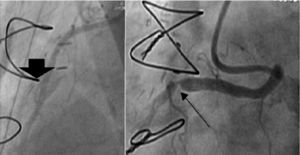
Coronary artery bypass grafting (CABG), with an arterial graft performed in our oldest patient (case 2), taking the precaution of creating a proximal stenosis in the anomalous RCA and thus avoiding competitive flow and occlusion of the graft (Figure 3);
- -
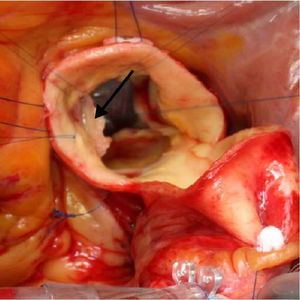
Unroofing,11 which is suitable for anomalous coronaries with long intramural segments, in which incision of the common wall of the aorta and anomalous coronary opens their intramural segment to the aortic lumen (Figure 4), undertaken in cases 5 and 7; and
- -
Neo-ostium creation, adopted when there is a long intramural segment of the anomalous coronary that crosses a commissure of the aortic valve, in which case unroofing would necessitate detaching the commissure, increasing the risk of later aortic regurgitation. In this case, instead of a complete unroofing procedure, only a partial incision is made in the common wall in the correct sinus, thus creating a coronary with two ostial openings (Figure 5); this was performed in patients 4 and 9.
All operated patients recovered without major complications. Median length of stay was 7 (5-21) days. Minor complications consisted of persistent atrial fibrillation and fever without focus in two patients. There was no in-hospital mortality.
Mean follow-up time is 90.3±19.7 months. One patient died 22 months after surgery, from a gall bladder carcinoma, resulting in a cumulative survival of 75±21% at 113 months after surgery (Figure 1). The other six are alive, asymptomatic in cardiac terms, and follow-up stress tests revealed no evidence of residual myocardial ischemia (Table 2).
DiscussionMost cases of sudden death in adults are caused by atherosclerotic ischemic heart disease. However, reports in young adults identify CAAs as the leading12 or second leading cause of SCD in the latter, after hypertrophic cardiomyopathy.13 ACAOS is a rare clinical entity, with a reported incidence of 0.06% to 0.9% for anomalous RCA and 0.025% and 0.15% for anomalous LCA in a cross-sectional imaging series,14 and 0.7% in an MRI-based screening series.15
In Portugal, three previous imaging studies documented incidences of coronary anomalies of 0.54%,4 0.68%5 and 2.69%.16 The latter, based on a consecutive series of 360 patients undergoing cardiac CT angiography, unlike the first two which were based on coronary angiography, included only symptomatic patients with a higher pretest probability of coronary disease, which may explain the higher incidence of coronary anomalies found.
The most serious anomaly is anomalous origin of the LCA from the pulmonary artery (also known as Bland-White-Garland syndrome). It is rare in adults, usually manifesting in infancy or early childhood, and without surgery about 90% of these children die within the first year of life.17 The principal CAA associated with SCD in young adults is ACAOS with an interarterial course,13 within the myocardial sulcus between the great arteries (intramyocardial) or within the anterior wall of the aorta between the great arteries (intramural).
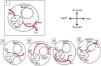
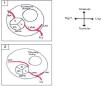
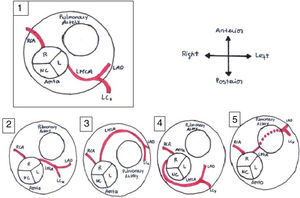
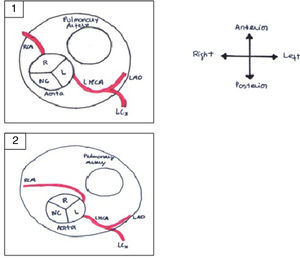
It is of major clinical importance which course is taken. Although retroaortic, prepulmonic and septal (subpulmonic) courses appear to be benign, an interarterial course carries a high risk for SCD and is often referred as a malignant course, most commonly an LCA arising from the right sinus of Valsalva (Figure 6).13,18,19 An LCA arising from the right sinus of Valsalva, although relatively rare (0.03%),20 is an important associated finding in patients with SCD.12 On the other hand, although less frequently associated with SCD, an RCA from the left sinus is more prevalent (0.1%)20 (Figure 7).
Anomalous origin of the left coronary artery in the right sinus. Course variations: (1) normal coronary anatomy; (2) interarterial course; (3) prepulmonary course; (4) retroaortic course; (5) subpulmonic course. L: left coronary sinus; LAD: left anterior descending artery; LCx: left circumflex artery; LMCA: left main coronary artery; NC: non-coronary sinus; R: right coronary sinus; RCA: right coronary artery. Adapted from Bienert et al.26.
Anomalous origin of the right coronary artery in the left sinus. (1) Normal coronary anatomy; (2) right coronary artery originating from the left sinus. L: left coronary sinus; LAD: left anterior descending artery; LCx: left circumflex artery; LMCA: left main coronary artery; NC: non-coronary sinus; R: right coronary sinus; RCA: right coronary artery. Adapted from Bienert et al.26.
Clinical manifestations range from asymptomatic to angina, myocardial infarction, heart failure, syncope, arrhythmias and sudden death.21 Myocardial ischemia in association with this anomaly can create an electrically unstable myocardial substrate predisposing to lethal ventricular tachyarrhythmias.22 Several potential mechanisms have been proposed to explain myocardial ischemia in patients with ACAOS: compression of the coronary artery between the aorta and pulmonary artery, particularly during exercise; an acute angle of coronary origin with a slit-like lumen and/or an ostial ridge as it arises from the aorta; spasm of the anomalous coronary artery, possibly as a result of endothelial injury; or a hypoplastic intramural coronary arterial course.13,22,23 Extrinsic compression of the left main coronary artery can occur in patients with severe pulmonary hypertension and enlarged pulmonary artery trunk.21 Accelerated development of atherosclerotic coronary disease has also been documented in some of these patients.24
Timely diagnosis remains a major clinical challenge, because of insufficient clinical suspicion, as well as the difficulties implicit in routine screening examinations or clinical testing for these malformations, which can be relatively difficult to diagnose since patients are often asymptomatic. Vague cardiovascular symptoms occur in 18-30% of them and SCD may be the first or only manifestation of underlying heart disease.5,22,25
Even in symptomatic patients, a correct diagnosis requires a very high index of clinical suspicion; 55%-93% of patients who die suddenly with a coronary anomaly have no forewarning, although about 10% are known to have had a pre-mortem cardiological evaluation for symptoms related to the anomaly.1 Echocardiography is a low-cost non-invasive method and has been effectively used to identify CAAs, but its sensitivity and specificity decrease considerably with increasing patient age, due to difficulties with the ultrasound window.26 The resting electrocardiogram (ECG) is a relatively effective screening test for cardiomyopathies such as hypertrophic cardiomyopathy, but it is almost always normal in the presence of congenital CAAs except with anomalous origin of the LCA from the pulmonary artery, and so the ECG provides little benefit in coronary anomaly screening, because the ischemia is transient.13,27 Other tests such as transesophageal echocardiography, cardiac magnetic resonance imaging and CT angiography are expensive and carry additional risks, but further assessment by the latter two techniques is recommended to determine the course of the anomaly and risk stratification.28 Multislice CT allows three-dimensional visualization of the coronary arteries with high spatial resolution, and may be the most promising imaging modality for diagnosing these anomalies. It has been reported that this technique may be superior to conventional angiography in defining ostial origin, proximal path of anomalous coronary branches and the presence of intramural hypoplasia.18,21
The decision whether to intervene can be difficult and is dependent on the type of lesion, the course of the coronary artery, its known association with SCD and any symptoms present at the time of diagnosis.20 For patients with obvious symptoms such as syncope or chest pain with exercise, documented exercise-induced ischemia or a documented episode of SCD, the decision regarding intervention is clear.
It is for the asymptomatic patient who has been diagnosed for other reasons that the decision is more difficult. For asymptomatic patients with an LCA arising from the right sinus with an interarterial course, the decision to intervene is more consensual29 on the basis of the large amount of myocardium at risk. However, for patients with an RCA from the left sinus the decision is not so clear. If it has an interarterial course and there is evidence of myocardial ischemia on a stress test, it should be corrected29 but, if the patient is asymptomatic and there is no evidence of ischemia, current guidelines accept clinical follow-up and avoidance of strenuous activity, as in our case 8, the young female former fire-fighter.
However, in the last 20 years, there have been at least 15 published reports of unexpected sudden death in previously asymptomatic patients with an anomalous RCA from the left sinus,30 not only during exercise31–33 but also at rest.34 Some of these patients had undergone previously normal cardiovascular examinations including ECGs, and in one case, despite a precise pre-mortem diagnosis, physical activity was not prohibited and the patient died during a soccer game.33 Furthermore, there are sufficient data suggesting a high risk of sudden death from exercise burden in young patients diagnosed with ACAOS with an interarterial course, whether the origin is right or left.33,35,36 In spite of this, controversially, other studies limited to middle-aged or older adults with ACAOS have reported a more benign clinical picture,37 with no deaths or similar major cardiovascular events in a matched cohort of patients without coronary anomalies.37,38 Major limitations of these studies are the presence of a majority of patients with RCA arising from the left sinus, a short follow-up time, and a possible selection bias toward low-risk patients who have survived their younger years.
Again, the absence of reported deaths in a large cohort of military recruits12 and only one sudden death in a review of two large prospective registries of young athletes in Italy and the USA22 suggest a more benign clinical picture. These deceptively contradictory data are well explained by the sample – the former report the risk of death of those living with ACAOS as opposed to the prevalence of ACAOS in patients who have already died.39 Whatever the real or theoretical risk, all the authors agreed that patients with an asymptomatic anomalous RCA should be prohibited from competitive sports, strenuous recreational activities or jobs imposing a high level of physical endeavor. While old or sedentary patients can more easily accept these restrictions, young or middle-aged adults might find them harder to follow, especially blue-collar or rural workers trying to find new employment. In these cases, we and others29,39 consider that surgery is justifiable and that the low surgical risk of the intervention outweighs the risk of sudden death, even if minimal, and its ominous consequences.
Intravascular ultrasound (IVUS) can support the indication for intervention by diagnosing the acute take-off of the anomalous coronary artery, initial segments of hypoplasia and lateral compression of the coronary wall by the aorta.39 With IVUS it is possible to quantify the coronary hypoplasia index (the ratio between the circumference of the smaller intramural intussuscepted segment and that of the more distal extramural vessel).2 Moreover, the lateral compression of the intramural segment, which results in a smaller area, particularly during systole, can be quantified by the ratio of the smallest to the largest diameter in an IVUS cross-section.2,39 A further workup should include nuclear stress testing, which is an important method to assess effort-induced ischemia and scars. It can also be used to establish the follow-up assessment after an intervention. Selective coronary angiography is indicated in situations when there is a need to exclude obstructive coronary disease and to assess the severity of congenital obstruction. Angelini and his group have proposed that IVUS should be used to establish acceptable selection criteria for interventional treatment.2 Unfortunately this diagnostic tool is not available in our institution, so the take-off of the coronaries is assessed by CT angiography.
Surgical techniques that have been used to repair this anomaly include:
- -
Ostial reimplantation is the preferred technique when the intramural segment crosses the aortic valve below the level of the commissures. Here, the proximal segment of the anomalous coronary artery is sectioned at the point where it exits from the aorta and is reimplanted in the appropriate sinus.14 It is also frequently used despite the concerns of some authors, who point out that the risk of coronary manipulation may theoretically exceed the natural risk of the lesion itself.40
- -
CABG has been disapproved, especially in young patients, because of the limited life-span of venous grafts and competitive flow in arterial grafts.41 However, CABG could play an important role in the management of older42 or high-risk patients, above all if performed off-pump, thus avoiding cardiopulmonary bypass, overcoming concerns about competitive flow by the creation of a proximal stenosis in the anomalous coronary, with intraoperative flow measurement of the arterial graft.
- -
Unroofing, the most commonly used technique, which consists of fenestration of the intramural portion of the anomalous artery, releasing the lumen of the coronary into the aorta. It is important to consider the relationship between the intramural coronary and the aortic valve commissures, because when the intramural segment travels below the level of the aortic commissures, unroofing of the intramural segment can damage the aortic valve and will increase the risk of aortic regurgitation months later.14,43,44
- -
Neo-ostium creation is performed when a long intramural segment of the anomalous coronary artery crosses an aortic valve commissure before exiting the wall. In this technique a new ostium is created in the exit sinus by unroofing the anomalous coronary at this level, avoiding manipulation of the aortic valve and reducing the risk of aortic regurgitation.14
- -
Pulmonary translocation,45 described in a few cases, is a complex operation consisting of enlarging the slit-like ostium and the proximal coronary, followed by lateral displacement of the pulmonary trunk and anastomosis to the left pulmonary artery, to prevent compression of the anomalous coronary between the great vessels. This has been specifically used in cases where there is a single coronary ostium and no intramural segment.14
Percutaneous stent implantation from the origin and covering the full extension of compression of the anomalous coronary artery, although technically challenging and carrying the problem of life-long antiplatelet therapy, has also been described, in both right46 and left47 ACAOS in older adults, although it is still the subject of debate.44
ConclusionACAOS with a course between the great vessels is a rare condition and most patients are asymptomatic. Surgery should be considered to prevent sudden death in patients with ACAOS plus symptoms suggestive of myocardial ischemia and also in asymptomatic patients, especially those with an LCA arising from the right sinus of Valsalva. The most controversial is the asymptomatic patient with an anomalous RCA from the left sinus. This is the more common lesion and although associated with SCD, it is unclear which subset of patients is at risk. Some authors postulate that a conservative approach, with beta-blockers and lifestyle measures in order to avoid strenuous exercise, may be adopted in the absence of symptoms or myocardial ischemia.20,26 Treatment should be individualized and the risk/benefit ratio should be assessed for every patient, but since the risks of surgery are minimal and the results are excellent,39–41,43–45 we agree that some of these patients should undergo surgical correction, whether or not symptoms are present, if they are young and engaged in physically strenuous jobs or hobbies.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to the Department of Cardiothoracic Surgery of Centro Hospitalar S. João for assistance in retrieving the data and for the clinical care of the patients; to Dr. Adelino Leite Moreira, for reading the paper; and to Dr. Jorge Almeida, for reading the paper and preoperative assessment of the patients.