Intravenous loop diuretics are an essential part of acute heart failure management; however, data to guide their use is sparse. Our aim was to compare continuous intravenous infusion of loop diuretics with intravenous bolus administration in terms of efficacy and adverse events in patients admitted with severe acute heart failure.
MethodsOver a period of three years, 110 patients were admitted to our cardiac intensive care unit with acute heart failure. Clinical, laboratory and prognostic parameters were compared according to the diuretic strategy used and mortality and readmission for acute heart failure during follow-up were analyzed.
ResultsPrevious medical history was similar in the two groups. At admission, the continuous infusion group met criteria for worse prognosis: lower systolic blood pressure (p=0.011), more severe renal injury (p=0.008), lower left ventricular ejection fraction (p=0.016) and higher incidence of restrictive pattern of diastolic dysfunction (p=0.032). They were more often treated with vasopressors (p=0.003), inotropes (p=0.010), renal support therapy (p=0.003) and non-invasive ventilation (p<0.001). They had longer hospitalizations (p=0.014) and a higher incidence of cardiorenal syndrome (p=0.009); however, at discharge, there were no differences in renal function between the groups. In-hospital mortality was similar, and during follow-up there were no differences in mortality or readmission for acute heart failure.
ConclusionsContinuous infusion was preferred in patients presenting with worse clinical status, in whom renal dysfunction was transiently worse. However, in-hospital mortality and creatinine at discharge were similar. Continuous infusion thus appears to counteract the initial dire prognosis of more unstable patients.
O uso de diuréticos da ansa é essencial no tratamento de doentes com insuficiência cardíaca aguda; contudo existe pouca evidência para guiar o seu uso. Neste trabalho comparámos a eficácia e efeitos adversos do uso de diuréticos em perfusão contínua com bólus em doentes com insuficiência cardíaca aguda.
MétodosAnálise de 110 doentes admitidos por insuficiência cardíaca aguda, ao longo de três anos, numa unidade de cuidados intensivos cardíacos. Parâmetros clínicos, analíticos e prognósticos foram comparados de acordo com a estratégia diurética utilizada. Realizado seguimento referente a mortalidade e reinternamento por insuficiência cardíaca aguda.
ResultadosA história médica prévia era semelhante. À admissão, o grupo da perfusão contínua reunia critérios de maior gravidade: pressão arterial sistólica mais baixa (p=0,011), lesão renal mais grave (p=0,008), menor fração de ejeção do ventrículo esquerdo (p=0,016) e maior incidência de padrão restritivo de disfunção diastólica (p=0,032). Foram tratados mais frequentemente com vasopressores (p=0,003), inotrópicos (p=0,010), terapêutica de suporte renal (p=0,003) e ventilação não invasiva (p<0,001). Tiveram internamentos mais prolongados (p=0,014) e maior incidência de síndrome cardiorrenal (p=0,009); contudo à alta não houve diferença na função renal entre os grupos. A mortalidade hospitalar foi semelhante; no seguimento não houve diferenças na mortalidade ou nos reinternamentos por insuficiência cardíaca.
ConclusõesA utilização de diuréticos da ansa em perfusão contínua foi preferida em doentes com critérios de maior gravidade à admissão. Transitoriamente apresentaram maior agravamento da função renal; contudo, a mortalidade hospitalar e a função renal à alta foram semelhantes. Assim, a perfusão contínua poderá ser uma opção em doentes mais instáveis.
acute heart failure
alanine transaminase
aspartate transaminase
blood urea nitrogen
cardiorenal syndrome
estimated glomerular filtration rate
heart failure
intravenous
length of hospital stay
left ventricular ejection fraction
Modification of Diet in Renal Disease
pulmonary artery systolic pressure
blood pressure
Hospitalizations for acute heart failure (AHF) have increased over time. Costs related to hospitalizations account for around 75% of the total cost of heart failure (HF) care.1 The prognosis of patients with AHF remains poor, with in-hospital mortality of 4%2 and a 30-day rehospitalization rate of 23%.3
Fluid retention and congestion are responsible for 90% of HF hospitalizations,2,4 and greater severity of congestion is associated with worse outcomes.5
Intravenous (IV) loop diuretics are a mainstay of the pharmacological treatment of AHF. Despite the ubiquitous use of these agents, uncertainties about appropriate dosing and overall safety profile persist.6,7 As a result, clinical practice varies widely across sites and countries with regard to both mode of administration and dosing.
Theoretically, continuous infusion of a loop diuretic, maintaining stable blood levels, should allow more sustained diuresis, avoiding large swings in intravascular volume and secondary neurohormonal activation. Continuous IV infusion with appropriate dosing may also prevent high or even toxic levels being reached, thereby causing fewer and less severe side effects.
Studies comparing the efficacy and safety of continuous and intermittent IV infusion of loop diuretics in AHF have yielded conflicting results and have been underpowered to address clinical questions, and thus no specific recommendations can be made.8
A recent randomized trial, the Diuretic Optimization Strategies Evaluation (DOSE) trial, challenged the existing clinical dogma concerning the optimal method of IV diuretic administration in hospitalized patients.9 However, criticisms have been made due to the low dosage of the diuretic regimens used. Also, more than 25% of patients included in this trial had left ventricular ejection fraction (LVEF) of 50% or greater, and exclusion criteria included systolic blood pressure (BP) <90 mmHg, serum creatinine >3.0 mg/dl (265 μmol/l) and need for IV vasodilators or inotropic agents,9 thus excluding the most critically ill patients with AHF.
In the present study, we sought to compare the clinical efficacy and safety of continuous and intermittent administration of IV furosemide in a less selected, real-world clinical setting.
MethodsPopulation and study designConsecutive patients admitted between January 2010 and November 2012 to a cardiac intensive care unit for AHF were selected. Diagnosis of AHF was made according to the definition in the European Society of Cardiology guidelines.10 Patients with acute coronary syndromes and end-stage renal disease on a regular hemodialysis program were excluded.
For this analysis, patients were divided into two groups according to the diuretic protocol selected by the admitting team: group A included patients receiving furosemide by continuous infusion; group B included patients who received only IV bolus furosemide therapy.
In group A furosemide infusion was titrated by the nursing team to achieve and maintain an hourly diuresis of 100–150 ml as a goal.
All patients had controlled fluid intake (1 l/day).
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Data collectionData collected from patients’ medical records included history of ischemic heart disease, hypertension, diabetes, chronic renal failure and/or obstructive sleep apnea; time-course and etiology of HF; admission heart rate, BP, and oxygen saturation; electrocardiogram; blood urea nitrogen (BUN), creatinine, troponin I, NT-pro-BNP, hemoglobin, sodium, potassium, total bilirubin, alkaline phosphatase, aspartate transaminase (AST), alanine transaminase (ALT) and cystatin C. Particular attention was paid to the evolution of renal function by determining maximum and discharge BUN and creatinine. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula, which has been shown to be the best method of indirect renal function assessment in patients with HF.11
The incidence of acute renal injury or cardiorenal syndrome (CRS), defined as an increase of ≥26.5 μmol/l in serum creatinine relative to the admission value,12,13 was also assessed.
The mean difference in serum creatinine at discharge compared to baseline was defined as a safety endpoint, chosen to reflect whether either strategy was associated with lasting effects on renal function.
All patients underwent transthoracic echocardiography and assessment of LVEF, diastolic function and pulmonary artery systolic pressure (PASP).
Treatments considered for the analysis were maximum daily furosemide dose; use of IV isosorbide dinitrate, dobutamine, dopamine, levosimendan and/or norepinephrine; renal support therapy and mechanical ventilation (both non-invasive and invasive).
In-hospital mortality, incidence of nosocomial infection and length of hospital stay (LOS) were assessed.
Readmission for AHF and mortality during follow-up were analyzed.
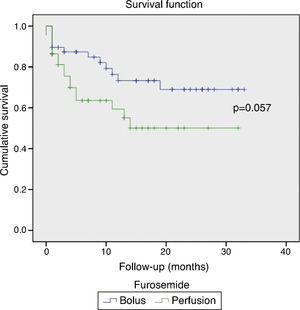
Statistical analysisThe statistical analysis was performed using SPSS for Windows, version 17.0. Categorical variables are expressed as a percentage of the total sample and compared using the chi-square test or Fisher's exact test, as appropriate. Continuous variables are expressed as mean ± standard deviation; the Student's t-test (two-tailed) was used to compare normally distributed variables and the Mann–Whitney U test to compare variables without a normal distribution. Survival analysis was performed with Kaplan–Meier curve analysis. For the purpose of this study, p values ≤0.05 were considered significant.
ResultsPatient populationA total of 110 patients were included in the study. Mean age was 68.5±14.5 years and 77% were male. Their baseline characteristics are shown in Table 1.
Baseline characteristics of the study population.
| History | |
| Hypertension (%) | 75 (68.2) |
| Diabetes (%) | 34 (30.9) |
| Chronic renal disease (%) | 47 (42.7) |
| Obstructive sleep apnea (%) | 15 (13.6) |
| Heart failure indices | |
| Previous admissions for AHF (%) | 46 (41.8) |
| LVEF, % | 32.4±12.1 |
| Ischemic etiology (%) | 28 (25.5) |
| NT-pro-BNP, pg/ml | 17400±22391 |
| Troponin I, ng/ml | 0.58±1.60 |
| Renal function | |
| Cystatin C, mg/l | 1.67±0.81 |
| Creatinine, μmol/l | 150.3±91.0 |
| BUN, mmol/l | 15.6±11.8 |
| eGFR, ml/min/1.73 m2 | 55.0±30.5 |
| eGFR <30 ml/min/1.73 m2 (%) | 25 (22.7) |
| Other admission laboratory values | |
| Hemoglobin, g/dl | 12.5±2.0 |
| Sodium, mmol/l | 137.8±4.9 |
| Potassium, mmol/l | 4.6±0.8 |
| Total bilirubin, μmol/l | 18.7±14.6 |
| Alkaline phosphatase, U/l | 113±59 |
| AST/ALT, U/l | 127±436/102±260 |
AHF: acute heart failure; ALT: alanine transaminase; AST: aspartate transaminase; BUN: blood urea nitrogen; eGFR: estimated glomerular filtration rate (Modification of Diet in Renal Disease formula); LVEF: left ventricular ejection fraction.
Less frequent etiologies of heart failure were valvular disease (17.3%) and alcohol abuse (13.6%). At least moderate systolic dysfunction (LVEF ≤40%) was observed in 79.0% of patients.
Continuous furosemide infusion was used in 56 (50.9%) patients, while IV bolus was chosen in the remaining 54 (49.1%). The mean maximum daily dose of furosemide was 163±126 mg.
Table 2 shows concomitant cardiovascular medical therapy and major in-hospital endpoints.
Concomitant cardiovascular medical therapy and major in-hospital endpoints.
| Concomitant cardiovascular medical therapy | |
| Isosorbide dinitrate (%) | 35 (35.8) |
| Levosimendan (%) | 49 (44.5) |
| Dobutamine (%) | 21 (19.1) |
| Dopamine (%) | 16 (14.5) |
| Noradrenaline (%) | 15 (13.6) |
| Other therapeutic strategies | |
| Non-invasive ventilation (%) | 48 (43.6) |
| Invasive ventilation (%) | 17 (15.5) |
| Renal support therapy (%) | 9 (8.2) |
| In-hospital endpoints | |
| Cardiorenal syndrome (%) | 71 (64.5) |
| Nosocomial infection (%) | 49 (44.5) |
| LOS, days | 13.6±10.8 |
| Mortality (%) | 17 (15.5) |
LOS: length of hospital stay.
No differences were found between the groups concerning gender (male: 78.6% vs. 75.9%; p=0.741), age (69.1±13.2 vs. 67.9±15.9 years; p=0.657), history of hypertension (64.3% vs. 72.2%; p=0.372), diabetes (35.7% vs. 25.9%; p=0.267), chronic renal disease (46.3% vs. 38.9%; p=0.473) or obstructive sleep apnea (14.3% vs. 13.0%; p=0.840). There were no differences concerning HF etiology (p=0.209, coronary artery disease being the most frequent in both groups [25.0% vs. 25.9%]), decompensating factors, or NYHA class on admission (NYHA IV: 67.9% vs. 66.7%; p=0.894). The incidence of atrial fibrillation at admission was also similar between groups (43.0% vs. 46.3%; p=0.432).
Group A more often presented with decompensated chronic HF (78.6% vs. 59.3%; p=0.028) rather than new-onset HF, and had more previous admissions for AHF (58.9% vs. 24.1%; p<0.001).
Table 3 shows some of the vital signs and laboratory data in the two groups at admission. No differences were found in respect to heart rate (94±28 vs. 96±29 bpm; p=0.618); hemoglobin (12.3±1.9 vs. 12.7±2.1 g/dl; p=0.337); potassium (4.6±1.0 vs. 4.6±0.7 mmol/l; p=0.992); troponin I (0.53±1.5 vs. 0.63±1.8 ng/ml; p=0.759); NT-pro-BNP (20 688±26 910 vs. 13 784±15 510 pg/ml; p=0.115); AST (131±454 vs. 123±421 U/l; p=0.930) or ALT (101±242 vs. 103±279 U/l; p=0.974).
Baseline vital signs and laboratory data: comparison between groups.
| Parameter | Group A (n=56) | Group B (n=54) | p |
|---|---|---|---|
| Vital signs | |||
| Systolic BP, mmHg | 112±28.3 | 128±37 | 0.011 |
| Oxygen saturation, % | 90.8±6.9 | 88.0±9.2 | 0.078 |
| Renal function | |||
| Cystatin C, mg/l | 1.96±0.92 | 1.42±0.63 | 0.008 |
| Creatinine, μmol/l | 164.2±105.8 | 135.9±70.7 | 0.103 |
| BUN, mmol/l | 17.7±12.3 | 13.4±10.9 | 0.057 |
| eGFR, ml/min/1.73 m2 | 52.2±32.0 | 57.8±28.8 | 0.343 |
| Other laboratory data | |||
| Sodium, mmol/l | 136.8±5.3 | 138.8±4.3 | 0.033 |
| Total bilirubin, μmol/l | 21.6±16.1 | 15.7±12.2 | 0.037 |
| Alkaline phosphatase, U/l | 125.0±68.8 | 99.7±42.8 | 0.024 |
BP: blood pressure; BUN: blood urea nitrogen; eGFR: estimated glomerular filtration rate (Modification of Diet in Renal Disease formula); Group A: continuous infusion; Group B: bolus administration.
Echocardiographic findings included lower LVEF in group A (30.2±11.2% vs. 34.8±12.6%; p=0.046), with a higher incidence of moderate to severe impairment of systolic function (LVEF <40%) (87.5% vs. 68.5%; p=0.016); they also more often presented type 3 diastolic dysfunction (restrictive filling pattern) (41.8% vs. 20.4%; p=0.032) and a trend towards higher PASP (51±17 mmHg vs. 45±13 mmHg; p=0.063).
Comparison of therapeutic strategiesTable 4 shows the main differences between groups concerning therapeutic strategies. No differences were found in the use of isosorbide dinitrate (25.0% vs. 38.9%; p=0.118) or invasive ventilation (17.9% vs. 13.0%; p=0.478).
Therapeutic strategies: main differences between the groups.
| Therapeutic strategy | Group A (n=56) | Group B (n=54) | p |
|---|---|---|---|
| Max. daily furosemide dose, mg | 246±127 | 78±31 | <0.001 |
| Dobutamine, % | 16 (28.6) | 5 (9.3) | 0.010 |
| Dopamine, % | 13 (23.2) | 3 (5.6) | 0.039 |
| Levosimendan, % | 30 (53.6) | 19 (35.2) | 0.052 |
| Noradrenaline, % | 13 (23.2) | 2 (3.7) | 0.003 |
| Renal support therapy, % | 9 (16.1) | 0 (0) | 0.003 |
| Non-invasive ventilation, % | 34 (60.7) | 14 (25.9) | <0.001 |
Group A: continuous infusion; Group B: bolus administration.
The incidence of CRS was higher in group A (76.8% vs. 51.9%; p=0.009). Table 5 and Figure 1 show the course of renal dysfunction during hospitalization in both groups. There were no differences in changes in creatinine (–4.0±76.4 vs. –0.3±52.7; p=0.784), BUN (2.3±9.5 vs. 0.6±8.3 mmol/l; p=0.361) or eGFR (–2.9±28.0 vs. –0.2±23.4 ml/min/1.73 m2; p=0.611) from admission to discharge.
Evolution of renal function during hospitalization.
| Renal function parameters | Group A (n=56) | Group B (n=54) | p |
|---|---|---|---|
| Maximum values | |||
| Creatinine, μmol/l | 234.2±144.6 | 165.7±91.3 | 0.004 |
| BUN, mmol/l | 26.2±13.5 | 18.1±11.7 | 0.001 |
| eGFR, ml/min/1.73 m2 | 33.5±16.8 | 45.6±22.6 | 0.002 |
| Discharge values | |||
| Creatinine, μmol/l | 141.4±86.0 | 131.9±70.9 | 0.557 |
| BUN, mmol/l | 17.0±9.4 | 13.7±9.4 | 0.091 |
| eGFR, ml/min/1.73 m2 | 53.9±21.7 | 59.4±27.6 | 0.284 |
BUN: blood urea nitrogen; eGFR: estimated glomerular filtration rate (Modification of Diet in Renal Disease formula); Group A: continuous infusion; Group B: bolus administration.
Group A had longer LOS (16.0±12.3 vs. 11.0±8.3 days; p=0.014) and a higher incidence of nosocomial infections (57.1% vs. 31.5%; p=0.005); however, in-hospital mortality was similar (19.6% vs. 11.1%; p=0.216).
Follow-upIn the first 30 days no differences were found in readmissions for AHF (16.3% vs. 14.6%; p=0.823) or mortality (15.9% vs. 4.2%; p=0.081).
These results persisted during a mean follow-up of 11.2±9.8 months, with a high rate of readmissions for AHF (58.1% vs. 45.8%; p=0.241), and mortality barely reaching statistical significance (38.6% vs. 25.0%; p=0.057). Figure 2 shows the Kaplan–Meier survival curves during follow-up.
DiscussionCongestion and volume retention are the hallmark of AHF, a condition with high morbidity and mortality for which there is no optimal treatment.
In current clinical practice, loop diuretics are the first-line therapy for AHF. Despite their familiarity, there remain many unanswered questions about their efficacy, outcomes, and safety. Determination of the optimal mode for diuretic therapy in the management of fluid volume in patients with AHF remains an ongoing issue.
In our study, which aims to describe a real-world severe AHF population, continuous infusion of furosemide was preferred by the attending team in patients presenting with more criteria of dire prognosis on admission. This tendency could relate to the perception that infusion allows for better monitoring and more stable urinary output, while avoiding falls in BP. Although there was greater deterioration in renal function during hospitalization in the continuous infusion group, at discharge there were no significant differences between the groups in creatinine or BUN. In theory, continuous infusion of a loop diuretic could maintain levels above the natriuretic threshold and provide more constant diuresis without swings in intravascular volume. A pooled analysis of prospective randomized controlled studies prior to 2004 concluded that continuous infusion was beneficial in terms of increased urine output and a better safety profile.8 However, the studies included in the analysis were quite small (254 patients in total) and heterogeneous in design, and the advantage disappeared when two studies using hypertonic saline were excluded.
Recent studies have failed to reach a consensus. Allen et al.14 found no advantage with continuous infusion, whereas Thomson et al.15 did find a significant increase in urine output with equivalent doses of furosemide administered via continuous infusion compared to intermittent boluses.
In fact, there is limited evidence to guide diuretic use, as reflected in practice guidelines10 in which diuretic therapy is given a class I recommendation, with a level of evidence based on a single randomized trial (the DOSE trial).9 This trial, in a well-matched baseline population, found no differences in patients’ global assessment of symptoms, net fluid loss, change in weight, renal safety, or LOS based on the mode of diuretic administration.
Although the DOSE trial was well-designed and is widely considered a landmark study, it excluded patients with systolic BP <90 mmHg and those requiring IV vasodilators or inotropic agents, and thus in our opinion does not reflect the severe AHF population of daily clinical practice.
Our study, albeit retrospective in design, has the advantage of including many patients with severe AHF. Compared with the DOSE trial our patients were older, had lower LVEF, and presented at admission with higher creatinine and NT-pro-BNP levels. The severity of the AHF episode was also reflected in the use of very high furosemide doses, need for renal support in 8.2%, and high use of inotropes, vasopressors, and mechanical ventilation. Hospital stay was complicated by CRS and nosocomial infection in around half of the population, which caused longer LOS than described in the literature. These factors may also explain our high in-hospital mortality.
Clinicians tended to choose IV furosemide infusion rather than bolus in patients presenting with decompensated chronic HF, rather than new-onset HF, in particular if BP was low. This coincided with other well-known parameters of poor prognosis, such as higher cystatin C levels, hyponatremia and hepatic/cholestatic jaundice. Not surprisingly, group A was more often treated with inotropes, vasopressors and non-invasive ventilation. The maximum daily furosemide dose was also significantly higher.
As expected for a more severe HF population, the incidence of CRS was higher, with higher peak creatinine values, requiring renal support therapy in 16.1% of patients. However, their improvement in renal function was such that they were discharged with similar eGFR to group B.
Unlike previous studies in which the endpoint was acute renal injury, in our study we used discharge renal function as a safety endpoint. By doing so we demonstrated that continuous infusion can be safe in critically ill patients. One possible explanation for the considerable improvement in renal function may be decreased fluctuations in intravascular volume due to relatively constant urine output associated with continuous infusion, as well as effective decongestion.
When treating patients with AHF, physicians should bear in mind that persistent congestion is itself a predictor of adverse outcomes; transient worsening of renal function may be an acceptable price to pay in order to achieve better short-term fluid removal and symptomatic relief.
It is of course impossible to determine whether it was the mode of administration or the higher dose of diuretics that was responsible for the similar results between the groups concerning the safety endpoint. Recent data9,16,17 have suggested that higher doses of diuretics are likely to be more efficacious in relieving congestion, at the cost of transient worsening of renal function, apparently with no long-term consequences. Previous observations linking high-dose diuretics with poor outcomes may reflect the severity of the illness (more advanced cardiac and renal disease) rather than the harmful effect of high doses. Whether repeated episodes of transient worsening of renal function may have permanent harmful effects in the long term cannot be determined from this study.
Also, the longer LOS in the continuous infusion group may have contributed to the similar outcomes in follow-up. The literature suggests that there may be a tradeoff in HF care between increased LOS and readmission rates.18,19 Many believe that longer LOS allows care teams more time to educate, prepare, and treat the patient.
Although the continuous infusion group contained patients with more severe episodes of AHF, there was no evidence of worse short- or long-term prognosis in terms of in-hospital mortality, readmissions for AHF or follow-up mortality. It should however be noted that the differences are close to statistical significance, and the population was probably too small to have the statistical power to detect differences.
In the authors’ opinion it would be difficult to prove that a single drug or strategy improves outcomes in patients with severe AHF. As shown in a recent trial,20 in the case of diuretics, a stepped algorithmic approach to dose titration and tailored addition of thiazide, vasodilators, and inotropic support may help guide pharmacological treatment and prevent diuretic resistance. Another study21 showed that a diuretic protocol can reduce admissions for AHF.
The authors believe that an algorithm for treatment that closely monitors response with constant fine-tuning of dosages allows safer and more effective administration of drugs with potentially dangerous adverse effects.
LimitationsOur study has a number of limitations that should be recognized.
First, the observational nature of the study means that doses and mode of administration of furosemide were at the discretion of the admitting physician. The lack of randomization to parallel treatment strategies also created two populations that were clearly different in severity on admission, which precludes firm conclusions regarding the superiority or inferiority of continuous infusion over intermittent bolus administration. We were unable to control for unknown factors that may have confounded our clinical outcomes, a known limitation of observational studies.
Second, body weight was not routinely measured on a daily basis, because a significant proportion of patients were bedridden due to the severity of AHF, and thus weight loss could not be used as a surrogate marker of better decongestion. However, recent studies indicate that total weight loss during hospitalization for AHF is not associated with improvements in recurrent HF or mortality.
Third, the only loop diuretic included in the analysis was furosemide, and so the conclusions cannot be extrapolated to the more potent loop diuretics torsemide and bumetanide. Although furosemide is the most widely used loop diuretic, it remains controversial whether the more potent loop diuretics should be first-line treatment. The rationale for the use of these diuretics is related to their potential superiority in achieving diuresis without increased adverse events.
Finally, we were unable to analyze the dose and the mode of administration separately. The higher doses of furosemide in the continuous infusion group probably also affected our results, contributing to better decongestion.
ConclusionsUntil more effective strategies for volume removal are available, appropriate diuretic administration is a major component in the care of AHF patients.
Loop diuretics should be used with constant dosage adjustment and with regular clinical assessment.
Although a conclusion has not yet been reached as to the best mode of IV diuretic administration, our non-randomized study certainly contributes to the evidence of efficacy and safety of very high doses of furosemide administered in continuous infusion to a seriously ill AHF population.
Renal function is both a limiting factor and an indication of the efficacy and safety of any treatment of heart failure. The fact that more severe AHF patients with worse admission renal function as assessed by cystatin C were discharged with similar creatinine levels to less severely ill patients is certainly reassuring for continuous IV furosemide protocols.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.











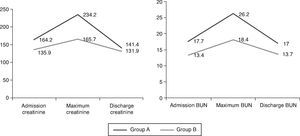 BUN: blood urea nitrogen; Group A: continuous infusion; Group B: bolus administration.'/>
BUN: blood urea nitrogen; Group A: continuous infusion; Group B: bolus administration.'/>