Patients referred for percutaneous transcatheter mitral valve repair using the MitraClip® system frequently have atrial fibrillation, which imposes additional challenges due to the need for oral anticoagulation. Left atrial appendage occlusion is currently regarded as a non-inferior alternative to anticoagulation in patients with non-valvular atrial fibrillation and both high thromboembolic and bleeding risk. Considering that both MitraClip implantation and left atrial appendage occlusion are percutaneous techniques that require transseptal puncture, it is technically attractive to consider their concomitant use.
ObjectivesWe aim to evaluate the feasibility of a combined approach with MitraClip implantation and left atrial appendage occlusion in a single procedure.
MethodsWe report the first case series regarding this issue, discussing the specific advantages, pitfalls and technical aspects of combining these two procedures.
ResultsFive patients underwent left atrial appendage occlusion with the Watchman® device followed by MitraClip implantation in the same procedure. All patients experienced significant reduction in mitral valve regurgitation of at least two grades, optimal occluder position, no associated complications and significant clinical improvement assessed by NYHA functional class (reduction of at least one functional class, with four patients in class I at one-month follow-up).
ConclusionIn selected patients rejected for surgical mitral valve repair, with atrial fibrillation and increased risk of bleeding and embolic events, a combined approach with MitraClip implantation and left atrial appendage occlusion in a single procedure is feasible, safe and effective.
Os doentes referenciados para reparação mitral percutânea, usando o sistema MitraClip, têm frequentemente fibrilhação auricular, com desafios adicionais devido à necessidade de anticoagulação. O encerramento percutâneo do apêndice auricular esquerdo é, atualmente, uma alternativa não inferior à anticoagulação em doentes com fibrilhação auricular não valvular, com elevado risco quer tromboembólico quer hemorrágico. Considerando que estas duas técnicas (MitraClip e encerramento percutâneo do apêndice auricular esquerdo) requerem punção transeptal, é tecnicamente atrativo considerar o seu uso concomitante.
ObjectivosAvaliar a exequibilidade de efectuar no mesmo procedimento implantação de MitraClip e encerramento percutâneo do apêndice auricular esquerdo.
MétodosDescrição da primeira série de casos sobre a aplicação destas duas técnicas num só procedimento, com análise das vantagens, dificuldades e aspetos técnicos.
ResultadosCinco doentes foram submetidos a encerramento percutâneo do apêndice auricular esquerdo com dispositivo Watchman, seguido de implantação de MitraClip no mesmo procedimento. Em todos os doentes verificou-se redução significativa do grau da regurgitação mitral em pelo menos dois graus, posição ótima do sistema de oclusão do apêndice, sem complicações associadas e com melhoria clínica significativa avaliada pela classe funcional de NYHA (redução de pelo menos uma classe funcional, com quatro doentes em classe funcional I no final do primeiro mês de seguimento).
ConclusãoEm doentes selecionados, recusados para reparação cirúrgica da valvular mitral, com fibrilhação auricular e risco elevado de eventos hemorrágicos e embólicos, uma abordagem combinada com implantação de MitraClip e encerramento percutâneo do apêndice auricular esquerdo num só procedimento é viável, segura e eficaz.
American College of Cardiology
atrial fibrillation
American Heart Association
European Society of Cardiology
Kidney Disease Outcomes Quality Initiative
left atrial appendage
left superior pulmonary vein
left ventricular ejection fraction
mitral regurgitation
mitral valve repair
New York Heart Association
oral anticoagulation
transesophageal echocardiography
Percutaneous transcatheter mitral valve repair (MVR) using the MitraClip® system is an emerging approach to treat selected cases of degenerative and functional mitral regurgitation (MR). Its safety and efficacy in high risk-patients has been consistently demonstrated, both in clinical trials and in real-world settings.1–3 The procedure has been recommended by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) since 2012 for patients with both primary and secondary severe MR at high surgical risk (class IIb, level of evidence C).4 Following approval by the US Food and Drug Administration (FDA) in 2013, the MitraClip was also recommended in the 2014 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for degenerative MR (class IIb, level of evidence B).5
Patients referred for MitraClip implantation frequently have concomitant atrial fibrillation (AF). These patients present high surgical risk due to the association of various comorbidities, as well as a frailty profile that represents increased risk of thromboembolic and bleeding events. These characteristics pose additional challenges due to the need for chronic oral anticoagulation (OAC).
Left atrial appendage (LAA) occlusion, either percutaneously or surgically, is currently regarded as an alternative to OAC in patients with non-valvular AF.6 Similarly to the MitraClip, this was as a result of two trials7,8 and several registries.9–12 The ESC guidelines issued a class IIb recommendation regarding this procedure,13 and it obtained FDA approval in 2015.
The frequent association of MR and AF has often led surgeons to excise the LAA during heart surgery.14 Considering that both MitraClip implantation and LAA occlusion are percutaneous techniques that require transseptal puncture and access to the left atrium, it is technically attractive to consider emulating the surgical approach percutaneously. With the exception of two single case reports, there are no other published data regarding the feasibility, safety and efficacy of a combined procedure.15,16 In this article, we report the first case series regarding this issue, discussing the specific advantages, pitfalls and technical aspects of combining these two procedures.
MethodsWe performed a retrospective single-center study of consecutive patients with severe MR and AF undergoing MitraClip device implantation plus LAA occlusion with the Watchman® device. Baseline clinical, echocardiographic, hemodynamic and procedural data were recorded retrospectively through reviews of hospital records. Echocardiographic assessment was repeated after the procedure, on the same day and at one-month follow-up. Acute and one-month results were analyzed.
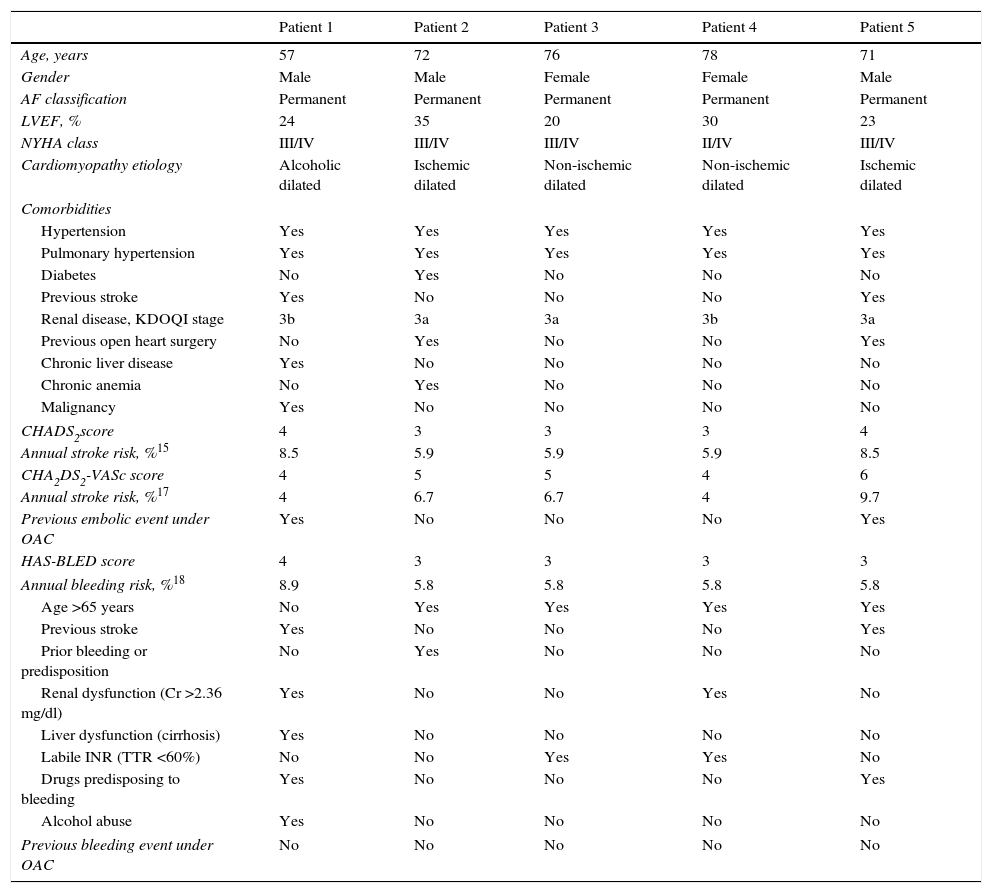
ResultsFive patients with severe MR and AF rejected for surgical MVR were referred for percutaneous MVR. After assessment by the interventional cardiology team, they were also selected for LAA occlusion on the basis of high thromboembolic risk combined with high bleeding risk. Table 1 shows the baseline patient characteristics.
Baseline patient characteristics.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age, years | 57 | 72 | 76 | 78 | 71 |
| Gender | Male | Male | Female | Female | Male |
| AF classification | Permanent | Permanent | Permanent | Permanent | Permanent |
| LVEF, % | 24 | 35 | 20 | 30 | 23 |
| NYHA class | III/IV | III/IV | III/IV | II/IV | III/IV |
| Cardiomyopathy etiology | Alcoholic dilated | Ischemic dilated | Non-ischemic dilated | Non-ischemic dilated | Ischemic dilated |
| Comorbidities | |||||
| Hypertension | Yes | Yes | Yes | Yes | Yes |
| Pulmonary hypertension | Yes | Yes | Yes | Yes | Yes |
| Diabetes | No | Yes | No | No | No |
| Previous stroke | Yes | No | No | No | Yes |
| Renal disease, KDOQI stage | 3b | 3a | 3a | 3b | 3a |
| Previous open heart surgery | No | Yes | No | No | Yes |
| Chronic liver disease | Yes | No | No | No | No |
| Chronic anemia | No | Yes | No | No | No |
| Malignancy | Yes | No | No | No | No |
| CHADS2score | 4 | 3 | 3 | 3 | 4 |
| Annual stroke risk, %15 | 8.5 | 5.9 | 5.9 | 5.9 | 8.5 |
| CHA2DS2-VASc score | 4 | 5 | 5 | 4 | 6 |
| Annual stroke risk, %17 | 4 | 6.7 | 6.7 | 4 | 9.7 |
| Previous embolic event under OAC | Yes | No | No | No | Yes |
| HAS-BLED score | 4 | 3 | 3 | 3 | 3 |
| Annual bleeding risk, %18 | 8.9 | 5.8 | 5.8 | 5.8 | 5.8 |
| Age >65 years | No | Yes | Yes | Yes | Yes |
| Previous stroke | Yes | No | No | No | Yes |
| Prior bleeding or predisposition | No | Yes | No | No | No |
| Renal dysfunction (Cr >2.36 mg/dl) | Yes | No | No | Yes | No |
| Liver dysfunction (cirrhosis) | Yes | No | No | No | No |
| Labile INR (TTR <60%) | No | No | Yes | Yes | No |
| Drugs predisposing to bleeding | Yes | No | No | No | Yes |
| Alcohol abuse | Yes | No | No | No | No |
| Previous bleeding event under OAC | No | No | No | No | No |
AF: atrial fibrillation; Cr: serum creatinine; INR: international normalized ratio; KDOQI: Kidney Disease Outcomes Quality Initiative; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; OAC: oral anticoagulation; TTR: time in therapeutic range.
Patient 1 was a 57-year-old man with alcoholic dilated cardiomyopathy, severely compromised left ventricular ejection fraction (LVEF) and severe functional MR. Cardiac resynchronization therapy and atrioventricular node ablation had already been performed. Concomitantly he had permanent AF, liver cirrhosis (Child-Pugh class B) without esophageal varices (with continued alcohol abuse), previous ischemic stroke under OAC, chronic renal disease (Kidney Disease Outcomes Quality Initiative [KDOQI] stage 3b), pulmonary and systemic hypertension, dyslipidemia and previous resection of colon cancer.
Patient 2 was a 72-year-old man with ischemic dilated cardiomyopathy and moderately depressed LVEF, with a cardiac defibrillator system implanted for primary prevention and severe functional MR with episodes of flash edema. He had previously undergone triple coronary artery bypass graft surgery. Other comorbidities were permanent AF, pulmonary and systemic hypertension, dyslipidemia, chronic kidney disease (KDOQI stage 3a), chronic iron deficiency anemia with intestinal angiodysplasia and previous smoking.
Patient 3 was a 76-year-old woman with non-ischemic dilated cardiomyopathy, severely depressed LVEF and severe functional MR, who had already undergone cardiac resynchronization therapy. She also had permanent AF with labile INR under warfarin (TTR <60%), pulmonary and systemic hypertension, dyslipidemia and chronic kidney dysfunction (KDOQI stage 3a), and chronic use of non-steroidal anti-inflammatory drugs due to osteoarticular disease.
Patient 4 was a 78-year-old man with non-ischemic dilated cardiomyopathy, severely depressed LVEF and severe functional MR. Concomitant comorbidities were permanent AF with labile INR under warfarin (TTR <60%), pulmonary and systemic hypertension, chronic kidney dysfunction (KDOQI stage 3b) and dyslipidemia.
Patient 5 was a 71-year-old man with ischemic dilated cardiomyopathy, severely depressed LVEF and severe functional MR. Concomitant comorbidities were previous ischemic stroke, permanent AF, systemic hypertension, chronic kidney dysfunction (KDOQI stage 3a) and dyslipidemia.
Overall, the patients had significant comorbidities, LVEF <35% and severe functional MR, and were severely symptomatic (NYHA class III or IV). They had a high risk of both thromboembolic and bleeding events, with mean CHA2DS2-VASc score of 4.5±0.6 and mean HAS-BLED score of 3.5±0.6. The mean annual stoke risk predicted by CHA2DS2-VASc was 6.0±1.4%, with a higher annual risk of major bleeding events predicted by HAS-BLED (6.2±2.9%).
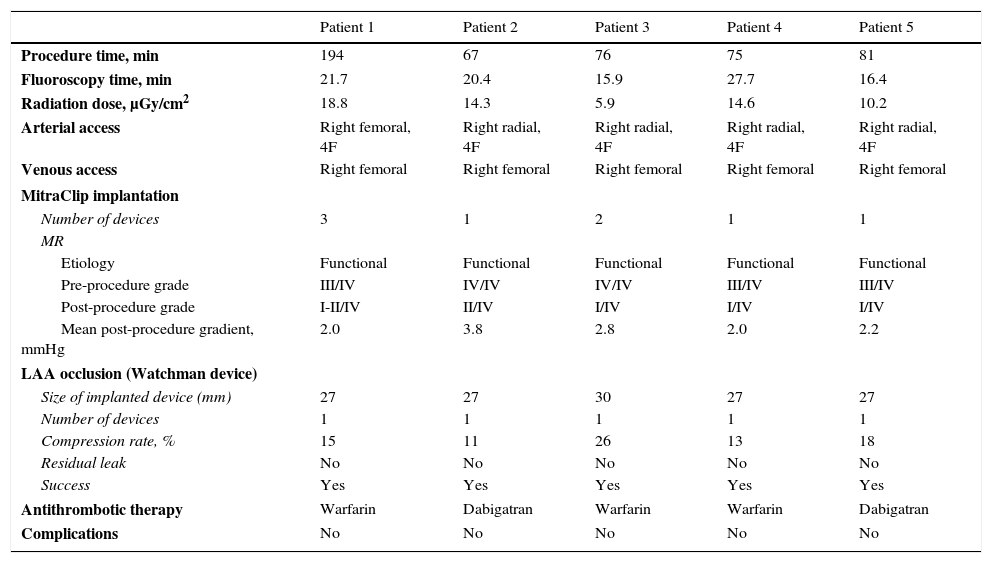
Procedural detailsThe procedure was performed under general anesthesia in the cardiac catheterization laboratory, with three-dimensional transesophageal echocardiography (TEE) guidance for MitraClip deployment and fluoroscopic guidance. In all cases LAA occlusion was performed first. Procedural details are displayed in Table 2.
Procedural details.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Procedure time, min | 194 | 67 | 76 | 75 | 81 |
| Fluoroscopy time, min | 21.7 | 20.4 | 15.9 | 27.7 | 16.4 |
| Radiation dose, μGy/cm2 | 18.8 | 14.3 | 5.9 | 14.6 | 10.2 |
| Arterial access | Right femoral, 4F | Right radial, 4F | Right radial, 4F | Right radial, 4F | Right radial, 4F |
| Venous access | Right femoral | Right femoral | Right femoral | Right femoral | Right femoral |
| MitraClip implantation | |||||
| Number of devices | 3 | 1 | 2 | 1 | 1 |
| MR | |||||
| Etiology | Functional | Functional | Functional | Functional | Functional |
| Pre-procedure grade | III/IV | IV/IV | IV/IV | III/IV | III/IV |
| Post-procedure grade | I-II/IV | II/IV | I/IV | I/IV | I/IV |
| Mean post-procedure gradient, mmHg | 2.0 | 3.8 | 2.8 | 2.0 | 2.2 |
| LAA occlusion (Watchman device) | |||||
| Size of implanted device (mm) | 27 | 27 | 30 | 27 | 27 |
| Number of devices | 1 | 1 | 1 | 1 | 1 |
| Compression rate, % | 15 | 11 | 26 | 13 | 18 |
| Residual leak | No | No | No | No | No |
| Success | Yes | Yes | Yes | Yes | Yes |
| Antithrombotic therapy | Warfarin | Dabigatran | Warfarin | Warfarin | Dabigatran |
| Complications | No | No | No | No | No |
LAA: left atrial appendage; MR: mitral regurgitation.
By protocol, right radial arterial access (4F sheath) was established for invasive blood pressure monitoring, except for one case in which access was via the right femoral artery (4F sheath) due to inability to obtain radial access. Venous access was via the right femoral vein (6F sheath) in all cases. After establishment of arterial and venous access, an intravenous unfractionated heparin bolus was administered (50% of the total recommended dose – 100 U/kg).
Transseptal punctureTransseptal puncture, guided by TEE, was performed through an 8F SL1 sheath (St. Jude Medical) with a BRK transseptal needle (St. Jude Medical). The puncture was performed aiming for the optimal position for MitraClip implantation.
The remaining dose of intravenous unfractionated heparin was then administered and an activated clotting time of 250-300 s was maintained throughout the procedure.
Once access to the left atrium was achieved, an Amplatzer stiff wire was advanced into the left superior pulmonary vein (LSPV).
Left atrial appendage occlusionFor LAA occlusion, a Watchman 14F double curve access sheath was used followed by angiography of the LAA with a pigtail catheter. The LAA closure device size was selected after assessing the following LAA features by TEE: ostium size and shape, and number of lobes and length in at least four TEE views (0°, 45°, 90° and 135°). These measurements were complemented by angiography of the LAA. The device was implanted under angiographic and echocardiographic (TEE) guidance using the standard technique. Criteria of success were achieved in all five patients. Only one device per patient was used, with appropriate stability, compression and sealing. No residual leaks or immediate complications were noted.
MitraClip procedureThe Watchman 14F double curve sheath was exchanged for a 24F MitraClip Guide Catheter, over an Amplatzer support wire positioned in the LSPV. A standard technique was used, under angiographic and TEE guidance.
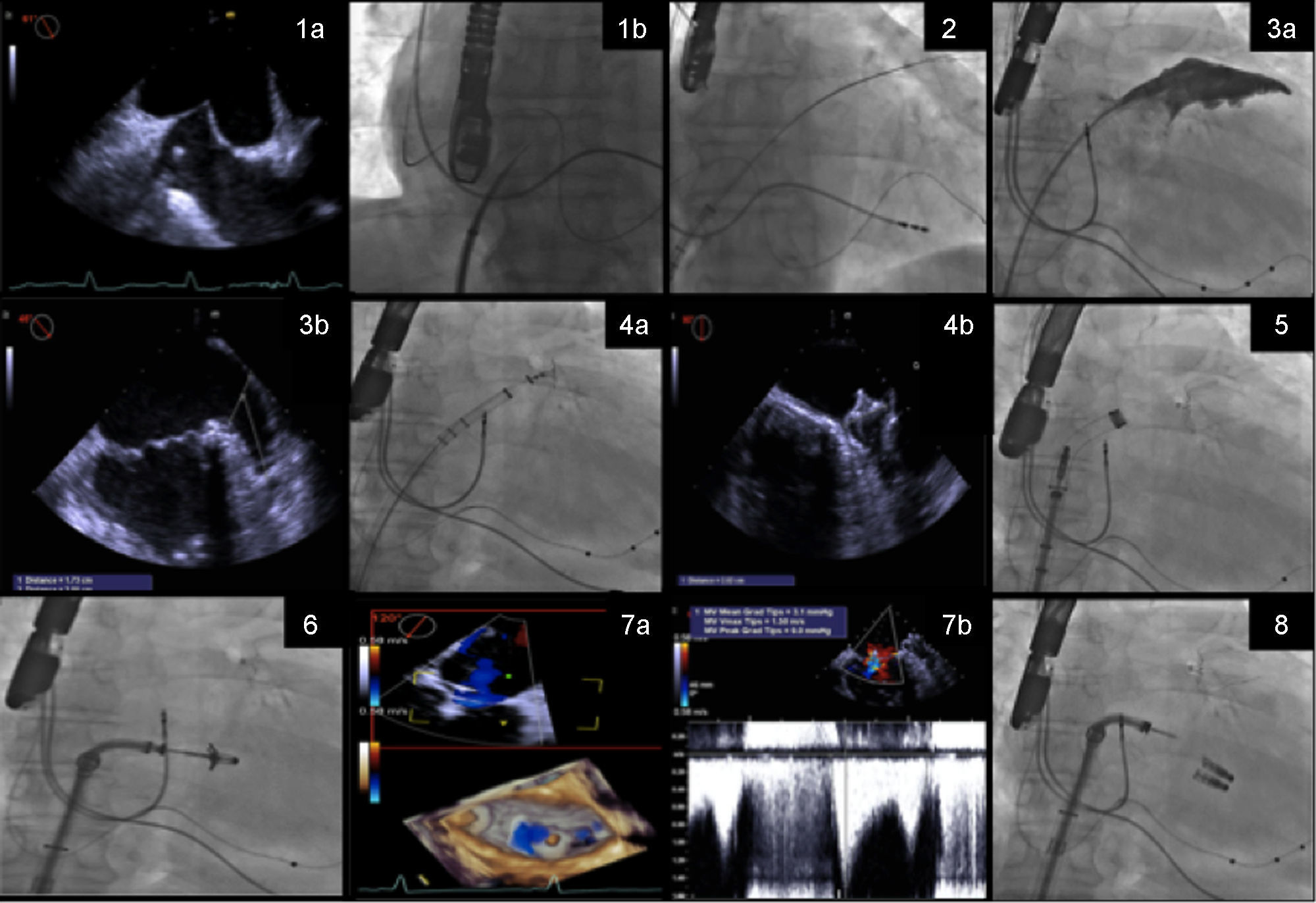
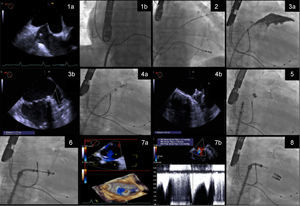
The procedure steps are summarized in Figure 1.
Procedure steps: (1) transseptal puncture guided by transesophageal echocardiography (TEE) (1a) and fluoroscopy (1b); (2) the Watchman 14F double curve sheath is advanced over an Amplatzer stiff wire positioned in the left superior pulmonary vein; (3) the left atrial appendage is measured by angiography (3a) and TEE (3b); (4) the Watchman is implanted guided by fluoroscopy (4a) and good apposition and absence of leaks and complications are confirmed by TEE (4b); (5) the Watchman 14F double curve sheath is exchanged for a 24F MitraClip guide catheter, over an Amplatzer support wire; (6) the clip delivery system is directed to the mid scallops of the anterior and posterior mitral valve leaflets, and guided by three-dimensional TEE, the clip is positioned above the origin of the mitral regurgitation (MR) jet; (7) leaflet insertion, MR reduction, and absence of significant mitral valve stenosis are confirmed by TEE (7a and 7b); (8) additional clips are deployed if necessary.
Three patients received only one clip, while the other two required two and three clips, respectively. All patients showed significant improvement in MR, three of them with residual grade I/IV regurgitation and the other two with grade II/IV. This goal was achieved without significant stenosis, mean final transvalvular gradient being 2.7±0.9 mmHg. No complications occurred. Of note, there was no interference of the MitraClip delivery system with the implanted Watchman device.
HemostasisIn all patients, the Perclose Proglide suture-mediated closure system (Abbott Vascular) was used for hemostasis of the femoral vein access site (two systems were pre-deployed, before insertion of the transseptal puncture sheath). Hemostasis of the arterial access site was performed by manual compression.
Follow-upAll patients were discharged 24-36 hours after the procedure. They started anticoagulation with warfarin or non-vitamin K antagonist oral anticoagulants, according to the choice of the attending cardiologist, with later transition to antiplatelet therapy after TEE follow-up (PROTECT AF strategy).10
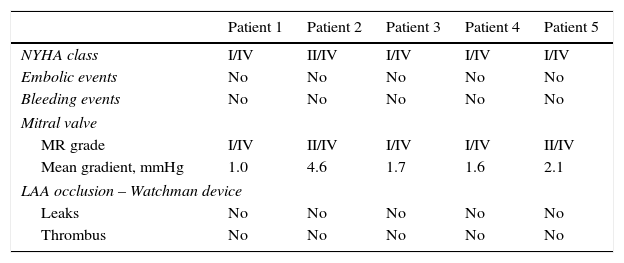
Patients underwent clinical and echocardiographic (TEE) follow-up one month after the procedure. Results were excellent in all patients, with clinical symptomatic improvement (NYHA class I-II/IV), significant MR reduction (without significant stenosis), optimal occluder position, no thrombus and no clinical events. The interatrial septum showed a small, not hemodynamically relevant, left-to-right shunt. Details are shown in Table 3. In a mean clinical follow-up of 243±70.7 days, the symptomatic improvement was maintained, without adverse events.
Results at one-month follow-up.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| NYHA class | I/IV | II/IV | I/IV | I/IV | I/IV |
| Embolic events | No | No | No | No | No |
| Bleeding events | No | No | No | No | No |
| Mitral valve | |||||
| MR grade | I/IV | II/IV | I/IV | I/IV | II/IV |
| Mean gradient, mmHg | 1.0 | 4.6 | 1.7 | 1.6 | 2.1 |
| LAA occlusion – Watchman device | |||||
| Leaks | No | No | No | No | No |
| Thrombus | No | No | No | No | No |
LAA: left atrial appendage; MR: mitral regurgitation; NYHA: New York Heart Association.
In this case series, we demonstrate the safety and effectiveness of combining percutaneous MVR using the MitraClip device and LAA occlusion with the Watchman device. It is should be noted that this approach produced excellent immediate and short-term results despite the severe cardiac and non-cardiac comorbidities of this cohort. These results are in agreement with the few published single case reports.15,16
Surgeons have long performed the combination of MVR, maze operation and LAA exclusion in patients with MR and AF, and LAA occlusion is recommended in patients with AF who undergo heart surgery.13 The LAA is easily accessible to the surgeon, the occlusion procedure is reasonably safe and it is believed to decrease embolic risk even in the presence of valvular AF. However, variability in surgical approaches means that remnants of the LAA are often left behind, which might explain the poor long-term results of this approach.14 During MitraClip implantation, the LAA is also easily accessible to the interventional cardiologist and the etiology of AF is non-rheumatic. In summary, the patient is anesthetized, the transseptal puncture has been performed and the LAA is easily accessible to catheter treatment. Percutaneous LAA occlusion has a solid scientific basis and good long-term results regarding both safety and efficacy. Therefore, combining MitraClip implantation and percutaneous LAA occlusion seems at least as appealing as combining surgical MVR and LAA excision.
From a clinical standpoint, patients referred for MitraClip implantation frequently present a profile suitable for LAA occlusion (AF with high thromboembolic and bleeding risk due to the comorbidities that excluded them from surgical MVR). Exposing these patients to two separate procedures would present several disadvantages. It is well recognized that transseptal puncture carries a small but important risk of significant complications.19 Furthermore, the use of large sheaths in two different locations may increase the risk of significant residual septal shunting. A single procedure involves a single transseptal puncture, thereby minimizing both risks. Also, both procedures require large sheaths placed in femoral veins. Combining the two procedures streamlines vascular access and reduces the risk of complications. Finally, overall fluoroscopy time may be reduced compared to two individual procedures, due to the common initial pathway of both techniques.
Our results seem to confirm these advantages, as there were no complications regarding any of the above-mentioned issues and total fluoroscopy time and radiation dosages were acceptable.
On the other hand, theoretically there are potential disadvantages. First, the high transseptal puncture for the MitraClip is less well suited for LAA occlusion. Second, overall procedure time may be prolonged, with an added risk of volume overload or hemodynamic instability, especially considering the severely depressed systolic function of many of these patients. We experienced no difficulties with either issue, and overall procedure time was acceptable (103.0±60.8 min). Nonetheless, acknowledging these pitfalls is essential for avoiding complications.
Another aspect of the technique that is the subject of debate is the appropriate sequence of procedures in this combined approach. Our team decided to perform LAA occlusion before MitraClip implantation based on the rationale of using sheaths with sequentially increasing diameters. We considered that this strategy would reduce the risk of bleeding at the access site and would impose less trauma on the atrial septum. Reversing the order of the procedures may have the advantage of eliminating the risk of interference of the MitraClip delivery system with the implanted Watchman device. However, we found that the presence of the Watchman device served as a useful anatomical reference during manipulation of the MitraClip delivery system. Performing the MitraClip implantation first would require an exchange for a shorter sheath compatible with the 14F Watchman delivery sheath, to avoid massive bleeding at the access site. Alternatively, the 24F could be exchanged directly for the 14F using the pre-deployed Perclose Proglide systems to close the orifice around the sheath. This approach may compromise final access occlusion success.
ConclusionIn selected patients at high risk for MR surgery who concomitantly present AF and an increased risk of both bleeding and embolic events, a combined approach with MitraClip and Watchman Occluder implantation in a single procedure is feasible, safe and effective, in both immediate and short-term follow-up, when undertaken by an experienced team.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNo specific funding grants were used regarding this study.
Conflicts of interestAna Rita G. Francisco has no conflicts of interest to declare; Eduardo Infante de Oliveira has received speaker/proctor honoraria and research grants from Boston Scientific and speaker honoraria from Abbott Vascular; Miguel Nobre Menezes has no conflicts of interest to declare; Pedro Carrilho Ferreira has received speaker honoraria and research grants from Boston Scientific; Pedro Canas da Silva has received speaker honoraria and research grants from Boston Scientific and Abbott Vascular.




 TEE) (1a) and fluoroscopy (1b); (2) the Watchman 14F double curve sheath is advanced over an Amplatzer stiff wire positioned in the left superior pulmonary vein; (3) the left atrial appendage is measured by angiography (3a) and
TEE) (1a) and fluoroscopy (1b); (2) the Watchman 14F double curve sheath is advanced over an Amplatzer stiff wire positioned in the left superior pulmonary vein; (3) the left atrial appendage is measured by angiography (3a) and 
