Atherogenic dyslipidemia is an important contributor to residual cardiovascular (CV) risk, but it is underdiagnosed and undertreated. This study aimed to assess the opinion of Portuguese experts to generate a consensus concerning the diagnosis and treatment of atherogenic dyslipidemia, as well as to contribute toward standardization of clinical practice in this disorder.
MethodsThe study consisted in the application of a questionnaire to an expert panel, following a modified Delphi methodology.
ResultsThe majority (88.4%) of the proposed items were found to be consensual. The expert panel recognized the importance of the atherogenic dyslipidemia phenotype, the role played by low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol as risk markers and therapeutic targets, the choice of statins as first-line lipid-lowering drugs, and the value of associating statins with fenofibrate as a means to reduce residual CV risk. However, the role played by triglycerides in CV risk and the therapeutic value of fibrates lacked consensus. Taking into consideration the state of the art and the opinions expressed in this study, the scientific committee developed a treatment algorithm aimed to improve the perception and treatment of atherogenic dyslipidemia.
ConclusionsThe experts involved in this study were shown to be familiar with the concept and the importance of atherogenic dyslipidemia. The few situations in which a consensus could not be found were mainly related to the interpretation and/or relevance of the available evidence.
A dislipidemia aterogénica contribui consideravelmente para o risco residual de doença cardiovascular (DCV); não obstante, esta patologia encontra-se subdiagnosticada e submedicada. O objetivo deste estudo foi analisar a opinião dos especialistas portugueses e gerar um consenso sobre o diagnóstico e o tratamento da dislipidemia aterogénica, bem como promover a uniformização da prática clínica neste contexto.
MétodosEste estudo consistiu na aplicação de um questionário a um painel de peritos seguindo uma metodologia Delphi modificada.
ResultadosA maioria dos itens (88,4%) foram consensuais. Os peritos inquiridos mostraram reconhecer a importância da dislipidemia aterogénica, o papel preponderante do colesterol de lipoproteínas de baixa densidade (c-LDL) e do colesterol total excluindo o das lipoproteínas de alta densidade (colesterol não HDL) na indicação do risco cardiovascular e na definição de objetivos terapêuticos, a eleição das estatinas como fármaco antidislipidémico de primeira linha, e a pertinência da sua associação com o fenofibrato para a redução do risco residual. Não obstante, o painel manifestou incerteza no que se refere ao papel dos triglicerídeos e ao valor terapêutico dos fibratos. Tendo como base a evidência disponível na literatura e as opiniões recolhidas neste estudo, a comissão científica elaborou um algoritmo de tratamento com o objetivo de promover a sensibilização e adequação da terapêutica a doentes com dislipidemia aterogénica.
ConclusõesOs inquiridos mostraram estar familiarizados com o conceito e com a importância da dislipidemia aterogénica; as poucas situações de ausência de consenso poderão estar relacionadas com uma diferente interpretação e/ou valorização da evidência científica disponível.
Despite substantial improvements in recent decades, according to the Organization for Economic Co-operation and Development (OECD), cardiovascular disease (CVD) remains the leading cause of death in most OECD member states, where it accounted for over a third of all deaths in 2015.1 In Portugal, diseases of the circulatory system were responsible for 32805 deaths (29.6% of total mortality) in 2016, while the number of years of potential life lost was 47923, corresponding to 546.6 per 100000 population and a mean number of years of life lost of 10.8.2
The risk factors for CVD are well known. They include age, gender, ethnicity, family history, smoking, dyslipidemia, hypertension, obesity and lack of exercise.3,4 Some of these factors are modifiable through lifestyle changes and appropriate medical therapy. However, in some cases risk for CVD persists. This residual risk is defined as the risk that remains after therapeutic goals have been achieved for low-density lipoprotein cholesterol (LDL-C), blood pressure and blood glucose.5
Atherogenic dyslipidemia, characterized by elevated levels of triglycerides (TG), very-low-density lipoprotein cholesterol (VLDL-C) and small dense low-density lipoprotein (LDL) particles and low levels of high-density lipoprotein cholesterol (HDL-C), contributes significantly to residual cardiovascular (CV) risk.5 Several studies have shown that the imbalance between elevated TG (which are proatherogenic, especially in the form of cholesterol-rich remnants) and reduced HDL-C increases CV risk independently of LDL-C levels.6,7 Detection and treatment of atherogenic dyslipidemia thus has an important role in the control of residual CV risk, particularly in groups in which this form of dyslipidemia is relatively common, such as patients with metabolic syndrome, obesity, or diabetes.
In epidemiological terms, the prevalence of atherogenic dyslipidemia is concerning. The European Study on Cardiovascular Risk Prevention and Management in Usual Daily Practice (EURIKA)8 enrolled 7641 patients with at least one CV risk factor but no history of CVD. Of this population, 20.8% had high TG (≥2.3mmol/l or 200mg/dl), 22.1% had low HDL-C (men: <1.0mmol/l or 40mg/dl; women: <1.3mmol/l or 50mg/dl), and 9.9% had both high TG and low HDL-C.8 Even so, approximately 55% of these patients were not taking any form of lipid-lowering therapy. The Portuguese arm of the Dyslipidemia International Study (DYSIS),9 which assessed the prevalence of persistent lipid abnormalities in patients receiving statins, included 916 patients, of whom 39.2% had elevated TG (>1.7mmol/l or 150mg/dl) and 22.2% had low HDL-C (<1.0mmol/l or 40mg/dl in men and <1.2mmol/l or 46mg/dl in women).10 In the subsequent DYSIS II, which included 10661 patients from 18 countries with stable coronary artery disease or acute coronary syndrome, median (interquartile range [IQR]) HDL-C levels among those taking lipid-lowering therapy were 42.0 (35.0–51.0) and 39.0 (33.0–47.0) mg/dl, while median TG levels were 120.0 (89.0–164.0) and 127.0 (94.0–177.0) mg/dl, respectively.11 Taken together, these results indicate a high prevalence of lipid abnormalities consistent with the phenotype of atherogenic dyslipidemia and suggest that the condition is both underdiagnosed and undertreated. Bearing in mind its importance in residual CV risk, it is urgent to invest in informing and educating health professionals, in order to improve the detection, monitoring and treatment of this form of dyslipidemia.
The aims of the Consenso Dislipidemia Aterogénica Portugal (CODAP, Consensus on Atherogenic Dyslipidemia in Portugal) study were to assess the opinion of Portuguese experts in order to generate a consensus concerning the definition, diagnosis and treatment of atherogenic dyslipidemia, and to contribute toward standardization of clinical practice in this disorder.
MethodsStudy designThe study consisted in the application of a questionnaire to an expert panel, following a modified Delphi methodology.12,13 Briefly, in this methodology, a survey is developed by a scientific committee and then distributed to a panel of experts invited to participate in the study. The initial responses (round 1) are analyzed by the committee and those questions on which there was no consensus are reformulated and returned to the panel together with the responses to the first round. This process is repeated until an agreed percentage of consensus is achieved.
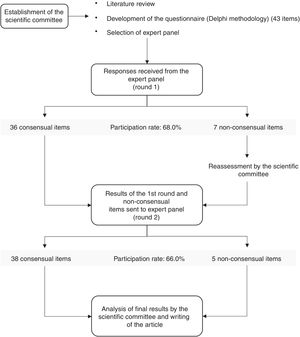
CODAP took place in four stages (Figure 1): (1) formation of the scientific committee and preparation of a questionnaire on different aspects of atherogenic dyslipidemia; (2) selection and invitation of members of the expert panel, made up of specialists in different areas of medicine; (3) responses to the survey by the expert panel in two rounds; and (4) analysis and discussion of the final results by the scientific committee. The selection of experts to be invited to form the panel was carried out in such a way as to ensure the greatest possible representativeness of physicians involved in the detection and treatment of atherogenic dyslipidemia, from four different specialties: cardiology, internal medicine, family and general medicine, and endocrinology. The invited physicians were carefully selected from lists of members of the Portuguese Society of Atherosclerosis and other relevant medical societies, all of whom were acknowledged experts in the field. In order to ensure equal representativeness in all specialties, a similar number of invitations (10–12) were sent to all groups, with the exception of endocrinology, which received 17 invitations because this specialty includes a subgroup of physicians with a particular interest in diabetology. The questionnaire was made available on an online platform and the responses were returned electronically.
Preparation of the questionnaire and definition of consensusThe questionnaire consisted of 43 statements on different aspects of atherogenic dyslipidemia: three on its definition, three on epidemiological aspects, seven on the impact of atherogenic dyslipidemia on CV risk, eight on methods of detection and diagnosis, seven on therapeutic goals, and 15 on treatment. These items were assessed by the expert panel on a Likert scale from 1 to 9 (1: completely disagree to 9: completely agree) and each option could be supplemented by comments entered into a free-text field. Consensus was defined as fulfillment of the following conditions: (1) the median of responses was between 1 and 3 (consensus in disagreement) or 7 and 9 (consensus in agreement); (2) the IQR of the median was 4 or less; and (3) the number of panel members voting outside the consensus range (1–3 or 7–9) was less than a third of the total votes.
ResultsIn the first stage, 50 physicians were invited to participate in the study: 12 specialists in cardiology, 11 in internal medicine, 10 in family and general medicine, and 17 in endocrinology. Thirty-four responses were obtained in the first round, a participation rate of 68.0% (Figure 1). Participation rates by specialty were 58.3% in cardiology, 90.9% in internal medicine, 80.0% in family and general medicine, and 52.9% in endocrinology. After the second and final round 33 responses were obtained, a final participation rate of 66.0% (Figure 1). The one panel member who did not respond to the second round was a specialist in cardiology, which thus had a final participation rate of 50.0%.
Of the 43 items on the questionnaire, there was consensus in agreement on 36 (83.7%) after the first round (Figure 1 and Table 1). Of the seven non-consensual items, three were reformulated and four were unchanged; two of the latter were accompanied by an explicit request for panel members to justify their response.
Results of the study questionnaire.
| Item | Median | IQR | % outside | Result |
|---|---|---|---|---|
| I. Definition | ||||
| I.1. Atherogenic dyslipidemia is an abnormality of lipid metabolism that is related to other lipid disorders including type 2 diabetes, obesity, metabolic syndrome and insulin resistance. | 9 | 8–9 | 5.71% | Consensus (round 1) |
| I.2. Atherogenic dyslipidemia is defined as a combination of low HDL-C (<40mg/dl [<1.0mmol/l) and <50mg/dl [<1.3mmol/l] in men and women, respectively), elevated triglycerides (≥150mg/dl or ≥1.7mmol/l), and an increased proportion of small dense LDL particles, in high-risk patients. | 9 | 8–9 | 5.71% | Consensus (round 1) |
| I.3. The higher the triglyceride level, the higher the proportion of small dense LDL particles. | 8 | 7–8 | 22.86% | Consensus (round 1) |
| II. Epidemiology | ||||
| II.1. In Portugal, atherogenic dyslipidemia is underdiagnosed and undertreated. | 8 | 7–9 | 20.00% | Consensus (round 1) |
| II.2. Atherogenic dyslipidemia is common in patients with type 2 diabetes, metabolic syndrome, obesity, chronic kidney disease and certain autoimmune diseases. | 8 | 8–9 | 5.71% | Consensus (round 1) |
| II.3. Residual cardiovascular risk is defined as the risk for cardiovascular events that persists in patients who have achieved therapeutic goals for LDL-C, blood pressure and blood glucose, in accordance with current standard treatment. | 8 | 7–9 | 2.86% | Consensus (round 1) |
| III. Cardiovascular risk | ||||
| III.1. In patients with atherogenic dyslipidemia, non-HDL-C is a better risk marker than LDL-C. | 8 | 6–8 | 28.13% | Consensus (round 2) |
| III.2. Patients with atherogenic dyslipidemia under statin therapy still have significant cardiovascular risk even when LDL-C is at target levels. | 8 | 6–8 | 25.71% | Consensus (round 1) |
| III.3. Reducing triglycerides improves cardiovascular risk in patients with atherogenic dyslipidemia and controlled LDL-C levels. | 7 | 5–8 | 37.50% | No consensus |
| III.4. Reducing non-HDL-C to levels no more than 30mg/dl higher than the target LDL-C level improves cardiovascular risk in patients with atherogenic dyslipidemia. | 7 | 7–8 | 22.86% | Consensus (round 1) |
| III.5. Non-HDL-C levels should be measured to determine cardiovascular risk in diabetic patients with elevated triglycerides. | 8 | 7–9 | 14.29% | Consensus (round 1) |
| III.6. Elevated triglycerides, as markers of triglyceride-rich lipoproteins such as remnant LDL, contribute to the pathogenesis of ischemic heart disease, as shown by recent genetic studies. | 7 | 6–8 | 31.43% | Consensus (round 1) |
| III.7. The cholesterol content of remnant particles appears to be a more important causal factor in cardiovascular risk than triglycerides. | 7 | 7–8 | 17.14% | Consensus (round 1) |
| IV. Detection and diagnosis | ||||
| IV.1. A high percentage of patients with atherogenic dyslipidemia under lipid-lowering therapy do not achieve the recommended therapeutic goals. | 8 | 7–9 | 8.57% | Consensus (round 1) |
| IV.2. All men aged over 40 years and all women aged over 50 years or post-menopausal should be screened for dyslipidemia, particularly when they have other vascular risk factors. | 9 | 8–9 | 8.57% | Consensus (round 1) |
| IV.3. Overall cardiovascular risk should be estimated (using a risk assessment tool such as SCORE) in all asymptomatic individuals aged over 40 years with no evidence of cardiovascular disease, diabetes, chronic kidney disease or familial hypercholesterolemia. | 9 | 8–9 | 14.29% | Consensus (round 1) |
| IV.4. LDL-C levels should be used in screening, risk assessment, diagnosis and treatment. | 8 | 7–9 | 11.43% | Consensus (round 1) |
| IV.5. Non-HDL-C should be considered a risk marker and should be measured, particularly in individuals with elevated triglycerides. | 8 | 7–9 | 17.14% | Consensus (round 1) |
| IV.6. HDL-C levels should be quantified before beginning treatment. | 8 | 7–9 | 20.00% | Consensus (round 1) |
| IV.7. Triglyceride levels should be taken into account in diagnosis and therapeutic decision-making. | 7 | 6–8 | 33.33% | No consensus |
| IV.8. ApoB levels can be used as an alternative to non-HDL-C. | 7 | 6–8 | 31.43% | Consensus (round 1) |
| V. Therapeutic goals | ||||
| V.1. The therapeutic goal should be an overall reduction in lipid levels rather than specific values of the different components of the lipid profile. | 5 | 2–7 | 62.50% | No consensus |
| V.2. In patients at very high cardiovascular risk, the goal should be LDL-C <70mg/dl (1.8mmol/l) or a reduction of at least 50% if baseline LDL-C is between 70 and 135mg/dl. | 9 | 8–9 | 2.86% | Consensus (round 1) |
| V.3. In patients at high cardiovascular risk, the goal should be LDL-C <100mg/dl (2.6mmol/l) or a reduction of at least 50% if baseline LDL-C is between 100 and 200mg/dl. | 9 | 8–9 | 5.71% | Consensus (round 1) |
| V.4. In patients at moderate or low cardiovascular risk, the goal should be LDL-C <115mg/dl (3.0mmol/l). | 9 | 7–9 | 0.00% | Consensus (round 1) |
| V.5. Pharmacological treatment of hypertriglyceridemia should be considered in high-risk patients with triglycerides >200mg/dl (2.3mmol/l). | 8 | 7–9 | 20.00% | Consensus (round 1) |
| V.6. A triglyceride level 500mg/dl is associated with a risk of pancreatitis, and pharmacological treatment to reduce triglyceridemia is required. | 9 | 8–9 | 5.71% | Consensus (round 1) |
| V.7. The therapeutic goal for non-HDL-C is easily calculated by adding 30g/dl (0.8mmol/l) to the therapeutic goal for LDL-C. | 9 | 7–9 | 11.43% | Consensus (round 1) |
| VI. Treatment | ||||
| VI.1. Lifestyle changes such as exercise and a healthy diet are an essential component of treatment of dyslipidemia, particularly atherogenic dyslipidemia. | 9 | 9–9 | 0.00% | Consensus (round 1) |
| VI.2. A multifactorial approach is recommended in patients with atherogenic dyslipidemia and type 2 diabetes, taking into account not only lipid profile but also control of blood pressure and of blood glucose and HbA1c levels. | 9 | 9–9 | 0.00% | Consensus (round 1) |
| VI.3. Family history should be taken into account. | 9 | 8–9 | 5.71% | Consensus (round 1) |
| VI.4. Statins are the first-line lipid-lowering therapy in patients with dyslipidemia and at high or very high cardiovascular risk. | 9 | 9–9 | 0.00% | Consensus (round 1) |
| VI.5. The statin and its dosage should be selected according to the extent of LDL-C reduction required to attain the therapeutic goal. | 9 | 9–9 | 2.86% | Consensus (round 1) |
| VI.6. Statins should be prescribed up to the maximum recommended or tolerated dose in order to attain the therapeutic goal. | 8 | 7–9 | 17.14% | Consensus (round 1) |
| VI.7. Ezetimibe can be combined with statins for greater reduction in LDL-C levels if the target level has not been achieved using statin monotherapy. | 9 | 8–9 | 2.86% | Consensus (round 1) |
| VI.8. In the pharmacological treatment of hypertriglyceridemia, fibrates are indicated to reduce triglyceride levels. | 8 | 7–9 | 20.00% | Consensus (round 1) |
| VI.9. Fibrates, particularly fenofibrate, should be used to treat residual cardiovascular risk associated with atherogenic dyslipidemia. | 7 | 5–9 | 43.75% | No consensus |
| VI.10. A combination of fenofibrate and statins can be considered in high-risk patients medicated with statins whose triglyceride levels remain above 200mg/dl (2.3mmol/l). | 8 | 6–8 | 28.57% | Consensus (round 1) |
| VI.11. Fenofibrate is the only fibrate recommended for combined therapy with statins. | 8 | 7–9 | 17.14% | Consensus (round 1) |
| VI.12. A combination of statin and fibrate can be used to treat residual cardiovascular risk associated with atherogenic dyslipidemia (i.e. elevated triglycerides and low HDL-C) in diabetic patients. | 8 | 7–8 | 22.86% | Consensus (round 1) |
| VI.13. A single-pill fixed-dose combination should be used to increase adherence to therapy in patients with atherogenic dyslipidemia. | 8 | 7–9 | 22.86% | Consensus (round 1) |
| VI.14. Fibrates have beneficial effects beyond reducing lipid levels, particularly by improving retinopathy and albuminuria in diabetic patients. | 7 | 6–8 | 40.63% | No consensus |
| VI.15. The results of the ACCORD trial demonstrated that treating atherogenic dyslipidemia in diabetic patients has benefits in terms of reducing cardiovascular risk. | 8 | 6–9 | 25.00% | Consensus (round 2) |
ApoB: apolipoprotein B; HbA1c: glycated hemoglobin; HDL-C: high-density lipoprotein cholesterol; IQR: interquartile range; % outside: percentage of responses outside the ranges 1–3, 4–6 or 7–9 (according to the median); LDL: low-density lipoprotein; LDL-C: low-density lipoprotein cholesterol; SCORE: Systematic COronary Risk Evaluation.
After the second round, consensus was reached on two of the seven items that were non-consensual in round 1 (Table 1): items III.1 and VI.15. The final rate of consensus was thus 88.4% (Figure 1 and Table 1), with consensus not being achieved on five items: III.3, IV.7, V.I, VI.9 and VI.14.
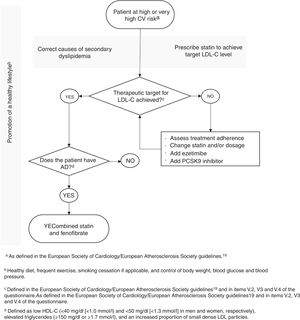
Based on the state of the art and on the responses to the questionnaire, the CODAP scientific committee developed a treatment algorithm for patients at high or very high risk for CV events, with the aim of standardizing clinical practice in the context of atherogenic dyslipidemia (Figure 2). According to this algorithm, after normalization of LDL-C levels in a patient medicated with statins and with a healthy lifestyle, the possibility of atherogenic dyslipidemia should be assessed. If its presence is confirmed, the scientific committee consider that the best currently available therapy is to associate fenofibrate with a statin.
DiscussionThe CODAP study shows that the opinions of Portuguese experts on the definition, importance, diagnosis and treatment of atherogenic dyslipidemia are largely consensual, with agreement on 88.4% of the items in the questionnaire. This figure is close to that reported in a similar study conducted in Spain, in which the rate of agreement was 87.4%.14 However, participation in the study was lower than expected, only 66% of responses to invitations being received from the two rounds. Although initially we sought to have the panel members distributed evenly among the specialties with the greatest clinical experience and involvement in the problems under study – cardiology, internal medicine, family and general medicine, and endocrinology (the latter including a subgroup of physicians with a particular interest in diabetology) – some specialties were considerably better represented in the final results than others. This limitation is not unique to this study, but is inherent to any methodology based on responses to a questionnaire: it is impossible at the outset to guarantee a specific number of responses or how they will be distributed among the population surveyed. Nevertheless, we consider that the responses obtained in our study are representative of physicians with an interest in the subject, whatever their specialty, and that the analysis and dissemination of these responses accomplishes the study’s initial aims.
Among the consensual subject areas, the best agreement was found for those related to the definition and epidemiology of atherogenic dyslipidemia, which were the only ones on which consensus was reached for all items. The lipid profile-based definition of atherogenic dyslipidemia – a combination of low HDL-C (<40mg/dl [<1.0mmol/l) and <50mg/dl [<1.3mmol/l] in men and women, respectively), elevated TG (≥150mg/dl or ≥1.7mmol/l), and an increased proportion of small dense LDL particles, in high-risk patients – was consensual, with agreement in the 7–9 range on the Likert scale among 94.3% of the panel members. This definition is generally accepted in the literature and was used in a recent consensus document derived from the opinions of European experts in CVD.5,15 However, it should be noted that the levels used in this definition can vary: recent articles published by the Spanish Society of Arteriosclerosis and a panel of European experts used a limit of 200mg/dl (2.3mmol/l) for TG.16,17
With regard to epidemiology, in agreement with the available evidence, there was consensus among panel members in recognizing that atherogenic dyslipidemia is particularly prevalent in patients with obesity, metabolic syndrome, type 2 diabetes and other metabolic disorders, and autoimmune diseases.5,17 Furthermore, 80% of participants agreed (in the 7–9 range) that atherogenic dyslipidemia is underdiagnosed and undertreated; this has been increasingly recognized in recent studies and appears to be true worldwide.8,10,18
There was consensus among the expert panel on the importance of LDL-C levels in screening, CV risk assessment, diagnosis and treatment, and on the need to formulate therapeutic goals for LDL-C that are personalized according to the individual patient’s risk level, as recommended in the clinical guidelines published jointly by the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS), among others.19 However, notwithstanding the undoubted importance of LDL-C in this context, the panel recognized the existence of residual risk even after therapeutic goals have been achieved for LDL-C. This realization is in line with the findings of the Residual Risk Reduction Initiative (R3i), which recently reviewed its first publications and, based on an extensive literature review, highlighted the imbalance caused by elevated TG and low HDL-C as a key contributor to residual CV risk.6,20 Even optimal medical therapy including proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, new drugs that significantly reduce LDL-C,21 appears to be unable to completely eliminate CV risk.
In line with their recognition of residual risk, the panel identified non-HDL-C as an important marker of CV risk, with 71.9% of members agreeing (in the 7–9 range) that non-HDL-C is a better risk marker than LDL-C in patients with atherogenic dyslipidemia. This view is fully supported by the literature, in which non-HDL-C, defined as the sum of LDL-C and remnant cholesterol (made up of VLDL-C and intermediate-density lipoprotein cholesterol [IDL-C]), is considered to be a more atherogenic cholesterol fraction than LDL-C,15,22–24 and is increasingly recognized as such by the medical community. Non-HDL-C now has an important place in the clinical guidelines: while the ESC/EAS, the International Atherosclerosis Society, and the Canadian Cardiovascular Society guidelines define non-HDL-C as a secondary or alternative target to LDL-C,19,25,26 the US National Lipid Association and the joint guidelines of the American Association of Clinical Endocrinologists and the American College of Endocrinology set parallel targets for LDL-C and non-HDL-C, and the UK National Institute for Health and Care Excellence sets non-HDL-C as the single therapeutic goal.29 Similarly, a recent consensus document developed by members of the main Portuguese medical societies states that non-HDL-C should be considered a better risk indicator than LDL-C, and should be treated as a secondary therapeutic target in the clinical management of dyslipidemia, especially in situations such as hypertriglyceridemia.30 It should also be noted that the expert panel in the CODAP study considered that apolipoprotein B (ApoB) levels can be taken as an alternative to non-HDL-C. Although they are not the same, non-HDL-C levels are closely related to those of ApoB, which explains their perceived equivalence and the fact that many of the above-mentioned guidelines also include therapeutic goals for ApoB. However, non-HDL-C is easily measured and is well known to all health professionals, which makes it a more attractive therapeutic target.
Curiously, the importance of TG in CV risk was not consensual among panel members. It had been assumed for decades that TG do not have an important role in the development of CVD. However, recent studies have demonstrated that high levels of TG and remnant cholesterol contribute directly to the pathophysiology of ischemic heart disease.31,32 It is now known that a TG level of 200–600mg/dl (2.3–6.8mmol/l) indicates an accumulation of highly atherogenic TG-rich remnant cholesterol.33,34 Three clinical trials, recently completed or under way, will help determine whether elevated TG contribute significantly to residual CV risk. In two of these trials omega-3 fatty acids (purified omega-3 fatty acids or ethyl esters of eicosapentaenoic acid) are used to lower TG,35,36 while the third uses a new fibrate, permafibrate.37
It should be noted that, while the panel recognized the importance of non-HDL-C in residual CV risk, they also agreed that TG also play a part, since TG levels are closely related to VLDL-C and IDL-C levels. Similarly, while there was no consensus on the value of TG levels in diagnosis and therapeutic decision-making, the panel agreed on the need to consider treating hypertriglyceridemia in high-risk patients with triglycerides >200mg/dl (2.3mmol/l), as recommended in the ESC/EAS guidelines.19 This suggests that the lack of consensus on questions related to TG may be due not to failure to appreciate their importance but to the complications and limitations of their quantification.30,38 TG levels vary considerably in a given individual, which makes them difficult to interpret. A recurrent question is whether they should be measured in a fasting or non-fasting state; recent studies have confirmed that postprandial TG levels may provide additional information on remnant cholesterol and are thus a complementary parameter to fasting TG.30 The fact remains that experts tend to favor non-HDL-C, which is easy to assess and levels of which provide an indirect measurement of TG. It is also possible that the currently available evidence is insufficiently robust to convince many specialists of the importance of TG. In this context, it is interesting to note that the role of TG as an independent CV risk factor was also not consensual in a survey of Spanish experts on atherogenic dyslipidemia.14
Another question on which consensus was not achieved was whether the therapeutic goal should be an overall reduction in lipid levels rather than specific values of the different components of the lipid profile. The lack of consensus on this point could have different interpretations. On one hand, it could demonstrate recognition of the importance of the HDL-C fraction, levels of which are inversely related to CV risk.39 On the other hand, it could be due to a more theoretical consideration, the fact that the aim of therapy is to reduce CV risk rather than to reduce levels of risk markers. The target values established in the guidelines for LDL-C and non-HDL-C are usually set in terms of the risk level of the individual patient.19,27,28 These issues may have raised doubts concerning the interpretation of this item in the questionnaire, leading to diverse responses and hence lack of consensus.
With regard to treatment, the panel were unanimous in recognizing the importance of healthy lifestyles and in the choice of statins as first-line lipid-lowering therapy in the treatment of dyslipidemia in patients at high or very high CV risk, as well as the addition of ezetimibe when target LDL-C levels are not achieved. These responses are mostly in line with the recommendations in the guidelines.19,25–29
The use of fibrates in the treatment of atherogenic dyslipidemia was also largely consensual. The panel recognized that fibrates are indicated for reducing TG levels and that associating fenofibrate with statins can be considered to treat hypertriglyceridemia in high-risk patients and to treat residual CV risk associated with atherogenic dyslipidemia in diabetic patients. Fenofibrate presents a low risk of drug interactions (it is a weak inhibitor of CYP2C19 and CYP2A6 and, at therapeutic levels, a moderate inhibitor of CYP2C9), and has only a minimal effect on the glucuronidation of statins; its safety has been demonstrated in intervention trials. However, there was no consensus on whether fibrates, particularly fenofibrate, should be used to treat residual CV risk associated with atherogenic dyslipidemia. This apparent discrepancy concerning the therapeutic value of fenofibrate in the context of atherogenic dyslipidemia reflects the literature: the two main randomized trials on the efficacy of fenofibrate – Action to Control Cardiovascular Risk in Diabetes (ACCORD)40 and Fenofibrate Intervention and Event Lowering in Diabetes (FIELD)41 – were considered overall to be negative, neither reaching statistical significance with regard to their primary outcome, first occurrence of non-fatal myocardial infarction, non-fatal stroke, or death from CV causes in ACCORD and coronary events (coronary heart disease death or non-fatal myocardial infarction) in FIELD. Nevertheless, some secondary results of FIELD clearly favor the use of fenofibrate, notably significant reductions of 24% in non-fatal myocardial infarction and of 11% in total CV events.41–45 Furthermore, the proportion of subjects who began treatment with other lipid-lowering drugs such as statins was higher in the placebo group (17% vs. 8% in the fenofibrate group, p<0.0001), which may have masked the benefit of the experimental drug. In ACCORD, analysis of a subgroup with TG>204mg/dl and HDL-C <34mg/dl showed that fenofibrate was associated with a significant 31% reduction in the incidence of CV events.43,45,46 This analysis was prespecified in the ACCORD trial protocol.47 In addition, an extended follow-up of participants in ACCORD, the ACCORDION study, suggested a possible effect of fenofibrate: five years after the original trial ended, patients with atherogenic dyslipidemia treated with fenofibrate continued to have a lower rate of CV events compared to placebo (hazard ratio 0.73; 95% confidence interval 0.56–0.95).48 Finally, in a meta-analysis that included FIELD, ACCORD and three other randomized trials, fibrates reduced CV events by 30% compared to placebo in individuals with atherogenic dyslipidemia (p<0.0001).49 All these results demonstrate the importance of fibrates, especially fenofibrate, in the treatment of patients with a phenotype consistent with atherogenic dyslipidemia. The addition of fibrates to first-line treatment in these individuals is already considered in some of the guidelines, albeit somewhat cautiously in view of the lack of randomized clinical trials specifically designed for populations with atherogenic dyslipidemia.15,19,25–27,50
Surprisingly, consensus was not achieved concerning the beneficial effects of fibrates on microvascular complications in diabetic patients, with only 59.4% of the experts surveyed in the range of agreement (7–9). However, the evidence appears to be clear on this point, showing that fibrates improve or at least slow the development of retinopathy and albuminuria.43–46 This results highlight the importance of dissemination of research and of continuing professional education for practicing physicians, in order to ensure that they remain up-to-date with current knowledge and that their clinical practice remains compatible with the best available evidence.
The treatment algorithm proposed by the scientific committee was based on the state of the art and on the responses of the expert panel. It is generally in agreement with the guidelines referred to above with regard to the importance of a healthy lifestyle as an essential component of therapy, the use of statins as first-line lipid-lowering therapy in patients at high or very high CV risk, assessment of LDL-C levels as the primary therapeutic target, and the need to review statin therapy and adherence to therapy, and possibly to add ezetimibe or PCSK9 inhibitors when LDL-C goals are not achieved.19,25–28,50 Adding fenofibrate to statins is, as stated above, considered in some of the guidelines, although the recommendation is not unequivocal. However, the committee considered that there is sufficient evidence to justify recommending this association as the best available alternative in individuals at high or very high CV risk and with atherogenic dyslipidemia which persists despite statin therapy.40–46,48,49 Moreover, fenofibrate is the only fibrate approved by the European Medicines Agency as an adjuvant to dietary changes or other non-pharmacological measures to treat mixed hyperlipidemia in patients at high CV risk, together with statins, when TG and HDL-C levels are not adequately controlled. In addition, unlike gemfibrozil, fenofibrate does not interact significantly with statins, which appears to explain the lower risk of muscle complaints with the combination of fenofibrate and a statin compared to gemfibrozil plus a statin.19
The development and implementation of a treatment algorithm for patients with atherogenic dyslipidemia is essential to standardize clinical practice and to ensure that this condition is properly identified and treated. This should reduce residual CV risk and hence associated morbidity and mortality. The Latin American Academy for the Study of Lipids (ALALIP) recently published a consensus document recommending a very similar treatment algorithm for patients with atherogenic dyslipidemia, with the difference of considering the addition of omega-3 fatty acids, as well as fibrates, to therapy when target non-HDL-C have not been achieved.18
ConclusionsThe Portuguese experts involved in this study were shown to be familiar with the importance of atherogenic dyslipidemia in the context of CV risk, particularly with regard to the definition of the condition and its association with other morbidities (especially metabolic disorders), the fact that atherogenic dyslipidemia is underdiagnosed and hence undertreated, the importance of LDL-C and non-HDL-C as risk indicators and therapeutic targets, the role of a healthy lifestyle as an essential component of treatment of dyslipidemia, the use of statins as first-line lipid-lowering therapy in individuals at high or very high CV risk, and awareness that combined fenofibrate and statins can be used to treat residual CV risk associated with atherogenic dyslipidemia in diabetic patients. However, despite the evidence in the literature, there was clearly some uncertainty about the role of TG as a risk marker and in therapeutic choices, and the therapeutic value of fibrates in the treatment of atherogenic dyslipidemia and in the prevention of microvascular complications in diabetic patients. While the first of these questions may arise because non-HDL-C is seen to be more important than TG, the doubts concerning fibrates appear to be mainly related to the interpretation and/or relevance of the available evidence.
In light of these findings, the scientific committee considers it essential to promote awareness of atherogenic dyslipidemia and associated CV risk, as well as dissemination of research and implementation of measures designed to improve identification and appropriate treatment of this condition. Thus, based on the information in the literature and on the responses to the questionnaire, the CODAP scientific committee present a proposed treatment algorithm that highlights the use of statins as first-line therapy, assessment of LDL-C levels as the primary therapeutic target, screening for the atherogenic dyslipidemia phenotype, and the addition of fenofibrate when it is diagnosed. It is hoped that this algorithm will function as a starting point for standardization and improvement of clinical practice in the treatment of atherogenic dyslipidemia in Portugal, and will thus help to reduce morbidity and mortality associated with CVD.
Conflicts of interestAMS has received honoraria and consulting fees from Amgen, AstraZeneca, Jaba-Recordati, Merck, Mylan, Novartis and Tecnimede. CA has received honoraria from Abbott, Amgen, Bial-Portela, JABA-Recordati, Merck Sharp & Dohme, Mylan, Sanofi-Regeneron and Tecnimede. JSD has received honoraria for lectures and clinical trials from Bial, Boehringer Ingelheim, JABA-Recordati, Merck Sharp & Dohme Portugal, Novo Nordisk, Novartis, Amgen, Sanofi and Tecnimede. PMS has received honoraria for lectures or consulting fees from Bayer, JABA-Recordati, Merck Sharp & Dohme Portugal, Kowa Pharmaceuticals, Novartis, Daiichi Sankyo, Amgen, Sanofi-Regeneron and Tecnimede.
This study was supported by Tecnimede, who contracted Springer Healthcare Communications for development and supervision of the project, writing up, and remuneration for the scientific committee and members of the expert panel for their intellectual contributions. Tecnimede had no influence on the design or development of the survey, selection of the expert panel, or interpretation of the results.
The authors would like to thank Catarina L. Santos, who assisted with medical editing, all the physicians who made up the expert panel, who gave up their time to respond to the questionnaire, (Abílio Malheiro, António Ferreira, Carlos Catarino, Davide Carvalho, Diogo Cruz, Elisabete Rodrigues, Elsa Gaspar, Evangelista Rocha, Francisco Araújo, Isabel Nazaré Santos, Isabel Palma, João Porto, João Raposo, Jorge Dores, José Augusto Simões, José Augusto Varandas, José Mendonça, José Pereira de Moura, José Silva Nunes, Luís Andrade, Luísa Maria Sá, Manuela Fiúza, Manuela Silva Melo, Maria Helena Ramos, Mariana Monteiro, Mário Lázaro, Nelson Rodrigues, Paulo Santos, Pedro Monteiro, Pedro von Hafe, Ricardo Fontes Carvalho, Roberto Palma Reis, Rui Carvalho and Vítor Ramalhinho).
Please cite this article as: Mello e Silva A, et al. CODAP: um consenso multidisciplinar sobre a definic¸ão, diagnóstico e tratamento da dislipidemia aterogénica em Portugal. Rev Port Cardiol. 2019;38:531–542.