The present study was designed to compare three-dimensional speckle tracking echocardiography (3DSTE)-derived left atrial (LA) volumetric, volume-based functional and strain parameters between patients with hypereosinophilic syndrome (HES) and matched controls.
MethodsA total of 10 HES patients and 19 age- and gender-matched healthy controls were included in the study. Complete two-dimensional Doppler echocardiography and 3DSTE were performed in all HES cases and controls.
ResultsSignificantly increased maximum (72.9±38.8 ml vs. 45.6±15.5 ml, p=0.01) and minimum (46.3±33.3 ml vs. 26.0±15.0 ml, p=0.03) LA volumes and LA volume before atrial contraction (62.0±36.0 ml vs. 36.5±16.6 ml, p=0.01) were found in HES patients compared to controls. Both peak global (18.3±6.7% vs. 25.6±9.0%, p=0.03) and mean segmental (22.2±6.0% vs. 31.0±12.1%, p=0.04) circumferential strains were significantly reduced in HES patients, suggesting decreased LA reservoir function.
ConclusionIncreased LA volumes can be demonstrated in HES patients, accompanied by reduced LA peak circumferential strain as assessed by 3DSTE, suggesting LA remodeling.
O presente estudo foi concebido para comparar parâmetros volumétricos auriculares esquerdos derivados por ecocardiografia tridimensional speckle tracking, parâmetros funcionais e strain baseados no volume entre doentes com síndrome hipereosinofílica e controlos equiparados.
MétodosUm total de dez doentes HES e de 19 controlos saudáveis, equiparados para idade e género, foram incluídos no estudo. O estudo completo ecocardiográfico bidimensional, Doppler e 3DSTE, foi realizado em todos os casos HES e controlos.
ResultadosEncontrou-se em doentes com HES, comparativamente com os controlos, um aumento significativo dos volumes auriculares esquerdos (72,9 ± 38,8 ml versus 45,6 ± 15,5 ml, p = 0,01), mínimo (46,3 ± 33,3 ml versus 26,0 ± 15,0 ml, p = 0,03) e volume auricular esquerdo antes da contração auricular (62,0 ± 36,0 ml versus 36,5 ± 16,6 ml, p = 0,01). Ambos strains circunferenciais pico, global (18,3 ± 6,7% versus 25,6 ± 9,0%, p = 0,03) e segmentar médio (22,2 ± 6,0% versus 31,0 ± 12,1%, p = 0,04) eram significativamente reduzidos em doentes HES, sugerindo diminuição de função auricular esquerda de reservatório.
ConclusãoO aumento de volumes auriculares esquerdos foi demonstrado nos doentes HES, sendo acompanhado por redução de strain circunferencial pico da aurícula esquerda conforme avaliado por 3DSTE, sugerindo remodelagem auricular esquerda.
Hypereosinophilic syndrome (HES) is characterized by persistently elevated eosinophil count (>1500 cells/ml) in peripheral blood for at least six months, and single- or multiple end-organ damage attributable to cytotoxic injury by eosinophils.1 In the literature the overall prevalence of cardiovascular involvement is 40–50% of HES patients.1–3 The early stage of cardiac involvement consists of eosinophilic infiltration, followed by an intermediate thrombotic stage, and finally a late fibrotic stage. An enlarged atrium with normal-sized left ventricle is a minor criterion for endomyocardial fibrosis.1 At present little is known about left atrial (LA) function in HES. Three-dimensional (3D) speckle tracking echocardiography (3DSTE) is a new non-invasive clinical tool for volumetric and strain analysis of the left atrium.4 The present study was designed to compare 3DSTE-derived LA volumetric, volume-based functional and strain parameters between HES patients and matched controls.
MethodsSubjectsA total of 10 HES patients were included in the present study. The diagnosis of HES was confirmed in all patients according to the available guidelines.5 One HES patient had a history of non-ST-elevation myocardial infarction; the others showed no significant cardiac alterations and were asymptomatic at the time of examination. Their results were compared to 19 age- and gender-matched healthy controls. Complete two-dimensional (2D) Doppler echocardiography and 3DSTE were performed in all HES patients and controls. The present work is a part of the Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases (MAGYAR-Path) Study (‘Magyar’ means ‘Hungarian’ in the Hungarian language), carried out at the 2nd Department of Medicine and Cardiology Center, Szeged, Hungary to validate 3DSTE-derived parameters, to examine their diagnostic and prognostic significance and to compare them to other known (patho)physiological variables in pathological cases. The protocol was approved by the institutional review board, conforming to the ethical guidelines of the 1975 Declaration of Helsinki, and each subject provided written informed consent.
Two-dimensional Doppler and tissue Doppler echocardiographyTransthoracic imaging was performed by experienced investigators using a 1–5 MHz PST-30SBP phased-array transducer in a Toshiba Artida™ cardiac ultrasound system (Toshiba Medical Systems, Tokyo, Japan). Complete two-dimensional echocardiography was undertaken with the patient in left lateral decubitus position, using both apical and parasternal views in all cases. Ejection fraction was measured in parasternal long-axis view by the Teichholz method.6 Color Doppler echocardiography was used to visually quantify degree of mitral regurgitation (MR). Tissue Doppler echocardiography was used to calculate the E/E′ ratio following pulsed Doppler-derived mitral inflow E/A measurements.
Three-dimensional speckle tracking echocardiography3DSTE datasets were acquired from an apical window using a 1-4 MHz matrix phased-array transducer (PST-25SX).4 Following optimization of gain setting, full volume mode was used over six consecutive cardiac cycles during a single breath-hold. The volume data were stored in raw data format for further analysis. LA quantifications were performed using 3D Wall Motion Tracking software, version 2.7 (Toshiba Medical Systems, Tokyo, Japan). Each 3D dataset was displayed in multiple planes, including apical two- (AP2CH) and four-chamber (AP4CH) views, and three short-axis views at different LA levels from the base to the apex. Several reference points on the LA endocardium were set by the examiner in AP2CH and AP4CH views. The first points were set at the edge of the septal mitral valve annulus (at the origin of the anterior mitral leaflet), then markers were placed in counterclockwise rotation around the LA to the lateral mitral valve annulus (to the origin of the posterior leaflet) in AP4CH view. During the studies the LA appendage and the pulmonary veins were excluded from the LA cavity. Measurements were performed first in AP4CH view, then in AP2CH view. After detection of the LA myocardial borders at the end-diastolic reference frame, the user could correct the LA shape, if necessary. 3D wall motion tracking was then automatically performed through the entire cardiac cycle.
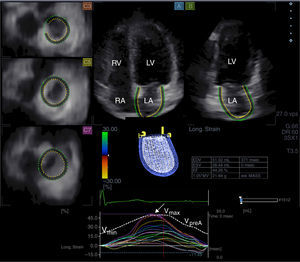
Three-dimensional speckle tracking echocardiography for left atrial volumetric measurementsFrom time curves of global LA volume changes, maximum (Vmax) and minimum (Vmin) LA volumes and LA volume before atrial contraction (VpreA) were measured from the 3D echocardiographic datasets just before mitral valve opening (end-systole), just before mitral valve closure (end-diastole) and at time of P wave on ECG (early diastole), respectively (Figure 1).7–10 LA function consists of three phases: the systolic reservoir phase, and the diastolic passive (conduit) and active emptying (booster pump) phases. To characterize these functions, stroke volumes and emptying fractions were calculated from the above-mentioned volumes:
Images from three-dimensional full-volume dataset showing left atrium: apical 4-chamber (A) and 2-chamber views (B) and short-axis views at basal (C3), mid- (C5) and superior (C7) left atrial level are demonstrated together with left atrial volumetric data and a three-dimensional cast of the left atrium. Time-segmental strain curves of all 16 left atrial segments and global left atrial volume changes throughout the cardiac cycle are also presented. White arrow: peak strain. LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle; Vmax, Vmin and VpreA: maximum and minimum left atrial volumes and left atrial volume before atrial contraction, respectively.
LA stroke volumes:
- -
Total atrial stroke volume (TASV): Vmax−Vmin (reservoir function)
- -
Passive atrial stroke volume (PASV): Vmax−VpreA (conduit function)
- -
Active atrial stroke volume (AASV): VpreA−Vmin (booster pump/active contraction function).
LA emptying fractions:
- -
Total atrial emptying fraction (TAEF): TASV/Vmax×100 (reservoir function)
- -
Passive atrial emptying fraction (PAEF): PASV/Vmax×100 (conduit function)
- -
Active atrial emptying fraction (AAEF): AASV/VpreA×100 (booster pump/active contraction function).
From the same 3D echocardiographic datasets, time curves of one-directional radial (RS), longitudinal (LS) and circumferential (CS) strains were also generated for each segment using the 16-segment model obtained for the left ventricle (Figure 1).10–12 Moreover, due to the ability of 3DSTE to calculate complex strains, area strain (ratio of endocardial area change during the cardiac cycle) and 3D strain (strain in the wall thickening direction, combination of one-directional strains) were also measured. On each time-segmental strain curve, peak strains representing characteristics of the reservoir phase of LA function were measured. Global strains were calculated by the software considering the whole LA, while mean segmental strains were obtained as the mean of strains of 16 segments. The software calculated these parameters automatically.
Statistical analysisContinuous variables are expressed as mean ± standard deviation. All statistical tests were two-sided and a p value <0.05 was regarded as statistically significant. Continuous variables were assessed by the unpaired Student's t test, while categorical variables were assessed by the chi-square test and Fisher's exact test. Correlations were established by calculation of Pearson correlation coefficients. The statistical analysis was performed with MedCalc software (MedCalc, Inc., Mariakerke, Belgium).
ResultsThe clinical data of each patient including organ involvement are presented in Table 1. Only one patient had a history of cardiac disease (non-ST-elevation myocardial infarction).
Clinical data of patients with hypereosinophilic syndrome.
| Case | Year of birth | Year of diagnosis | Organ involvement | Hepatomegaly/splenomegaly | Cardiac disease |
|---|---|---|---|---|---|
| 1 | 1961 | 2013 | Duodenal eosinophilia | Splenomegaly | - |
| 2 | 1938 | 2009 | - | - | - |
| 3 | 1966 | 2010 | - | - | - |
| 4 | 1945 | 2011 | Tissue eosinophilia | - | - |
| 5 | 1936 | 2013 | - | - | NSTEMI (2013) |
| 6 | 1940 | 2009 | Eosinophilic dermatitis | - | - |
| 7 | 1966 | 2011 | Sensory-motor neuropathy, pulmonary involvement, sural nerve granulomatous necrotizing vasculitis | Splenomegaly | - |
| 8 | 1954 | 2013 | - | - | - |
| 9 | 1961 | 2002 | - | - | - |
| 10 | 1941 | 2014 | Asthma, vasculitis | - | - |
NSTEMI: non-ST-elevation myocardial infarction.
The laboratory findings were as follows (HES vs. controls): red blood cell count: 4.2±0.5 T/l vs. 4.3±0.4 T/l (p=0.94); hemoglobin: 126.7±18.8 g/l vs. 130.1±10.2 g/l (p=0.86); platelet count: 276.3±176.7×109/l vs. 282.4±158.2×109/l; hematocrit: 36.9±5.5% vs. 37.8±4.9%; white blood cell count: 16.7±5.8×109/l vs. 6.8±1.2×109/l (p=0.02); eosinophil ratio: 49.0±16.6% vs. 3.2±2.3% (p=0.001); and absolute eosinophil count: 8.7±4.8×109/l vs. 0.4±0.1×109/l (p=0.001). No correlations could be demonstrated between any of the laboratory findings and 2D echocardiographic and 3DSTE data in this patient population.
The routine two-dimensional echocardiographic data are presented in Table 2. None of the controls or HES patients showed grade >1 mitral or tricuspid regurgitation. Only LA diameter and interventricular septal thickness showed significant differences between HES patients and controls.
Clinical and two-dimensional echocardiographic characteristics of patients with hypereosinophilic syndrome and controls.
| HES patients (n=10) | Controls (n=19) | p | |
|---|---|---|---|
| Risk factors | |||
| Age (years) | 58.1±13.1 | 51.2±12.6 | 0.18 |
| Male gender (%) | 7 (70) | 11 (58) | 0.69 |
| Body surface area (m2) | 1.78±0.13 | 1.77±0.12 | 0.92 |
| Body mass index (kg/m2) | 26.7±1.1 | 25.3±1.0 | 0.09 |
| Two-dimensional echocardiography | |||
| LA diameter (mm) | 39.5±3.5 | 32.9±2.2 | 0.0005 |
| LVEDD (mm) | 53.0±11.6 | 47.4±4.6 | 0.07 |
| LVEDV (ml) | 120.0±46.8 | 104.5±22.4 | 0.24 |
| LVEDV/BSA (ml/m2) | 67.2±25.3 | 59.4±14.3 | 0.42 |
| LVEDV/BMI (ml/[kg/m2]) | 4.5±1.9 | 4.1±0.9 | 0.58 |
| LV end-systolic diameter (mm) | 36.1±12.5 | 31.1±4.6 | 0.13 |
| LV end-systolic volume (ml) | 47.8±25.0 | 37.7±11.0 | 0.14 |
| LVESV/BSA (ml/m2) | 26.9±13.9 | 21.6±7.5 | 0.32 |
| LVESV/BMI (ml/[kg/m2]) | 1.8±0.9 | 1.5±0.5 | 0.39 |
| Interventricular septum (mm) | 11.0±0.7 | 8.7±1.7 | 0.0003 |
| LV posterior wall (mm) | 9.5±1.2 | 9.7±2.5 | 0.81 |
| LV ejection fraction (%) | 60.5±12.4 | 63.9±6.5 | 0.33 |
| E/A ratio | 1.88±0.16 | 1.43±0.28 | 0.05 |
| E/E′ ratio | 10.2±3.4 | 6.53±1.15 | 0.04 |
BMI: body mass index; BSA: body surface area; HES: hypereosinophilic syndrome; LA: left atrial; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVESD: left ventricular end-systolic diameter; LVESV: end-systolic volume.
The 3DSTE data are presented in Table 3. Significantly increased maximum (p=0.01) and minimum (p=0.03) LA volumes and LA volume before atrial contraction (p=0.01) and elevated total (p=0.02) and active (p=0.005) atrial stroke volumes characterizing LA reservoir and booster pump functions were found in HES patients as compared to controls (Table 3). All emptying fractions did not differ between groups. Both global and mean segmental peak CS were significantly reduced in HES patients, suggesting decreased LA reservoir function (Table 4).
Comparison of three-dimensional speckle tracking echocardiography-derived volumetric and volume-based left atrial functional parameters of patients with hypereosinophilic syndrome and controls.
| HES patients (n=10) | Controls (n=19) | p | |
|---|---|---|---|
| Calculated volumes | |||
| Vmax (ml) | 72.9±38.8 | 45.6±15.5 | 0.01 |
| Vmax/BSA (ml/m2) | 41.8±25.0 | 26.0±9.7 | 0.03 |
| Vmax/BMI (ml/[kg/m2]) | 2.8±1.5 | 1.8±0.6 | 0.03 |
| Vmin (ml) | 46.3±33.3 | 26.0±15.0 | 0.03 |
| Vmin/BSA (ml/m2) | 26.8±21.4 | 14.9±9.7 | 0.05 |
| Vmin/BMI (ml/[kg/m2]) | 1.7±1.2 | 1.0±0.6 | 0.05 |
| VpreA (ml) | 62.0±36.0 | 36.5±16.6 | 0.01 |
| VpreA/BSA (ml/m2) | 35.7±23.1 | 20.9±10.4 | 0.03 |
| VpreA/BMI (ml/[kg/m2]) | 2.3±1.4 | 1.4±0.7 | 0.03 |
| Stroke volumes | |||
| TASV (ml) | 26.6±8.5 | 19.6±6.4 | 0.02 |
| TASV/BSA (ml/m2) | 15.0±4.9 | 11.1±3.6 | 0.04 |
| TASV/BMI (ml/[kg/m2]) | 1.0±0.3 | 0.8±0.3 | 0.12 |
| PASV (ml) | 10.9±8.2 | 9.1±5.0 | 0.47 |
| PASV/BSA (ml/m2) | 6.1±4.6 | 5.1±2.8 | 0.61 |
| PASV/BMI (ml/[kg/m2]) | 0.4±0.3 | 0.4±0.2 | 0.78 |
| AASV (ml) | 15.7±5.1 | 10.5±4.0 | 0.005 |
| AASV/BSA (ml/m2) | 8.9±2.9 | 5.9±2.3 | 0.01 |
| AASV/BMI (ml/[kg/m2]) | 0.6±0.2 | 0.4±0.2 | 0.03 |
| Emptying fractions | |||
| Total atrial EF (%) | 40.0±10.5 | 45.0±12.9 | 0.29 |
| Passive atrial EF (%) | 15.9±11.7 | 21.4±10.8 | 0.21 |
| Active atrial EF (%) | 28.6±7.8 | 30.5±9.5 | 0.58 |
AASV: active atrial stroke volume; BMI: body mass index; BSA: body surface area; EF: emptying fraction; HES: hypereosinophilic syndrome; LA: left atrial; PASV: passive atrial stroke volume; TASV: total atrial stroke volume; Vmax: maximum left atrial volume; Vmin: minimum left atrial volume; VpreA: left atrial volume before atrial contraction.
Comparison of three-dimensional speckle tracking echocardiography-derived peak global and mean segmental strain parameters of patients with hypereosinophilic syndrome and controls.
| HES patients (n=10) | Controls (n=19) | p | |
|---|---|---|---|
| Global strain parameters | |||
| Radial strain (%) | –17.7±7.7 | –15.7±11.6 | 0.64 |
| Circumferential strain (%) | 18.3±6.7 | 25.6±9.0 | 0.03 |
| Longitudinal strain (%) | 21.0±6.2 | 22.3±8.7 | 0.68 |
| 3D strain (%) | –10.1±5.0 | –9.3±9.0 | 0.81 |
| Area strain (%) | 41.2±13.8 | 50.7±20.4 | 0.20 |
| Mean segmental strain parameters | |||
| Radial strain (%) | –20.6±6.1 | –19.5±8.1 | 0.70 |
| Circumferential strain (%) | 22.2±6.0 | 31.0±12.1 | 0.04 |
| Longitudinal strain (%) | 21.8±6.4 | 25.6±7.5 | 0.18 |
| 3D strain (%) | –14.7±4.3 | –13.7±6.7 | 0.67 |
| Area strain (%) | 45.6±13.1 | 58.3±21.7 | 0.10 |
3D: three-dimensional; HES: hypereosinophilic syndrome.
Cardiac manifestations are the major cause of morbidity in HES and follow three stages. The first, an acute necrotic stage, is due to infiltration of eosinophils in the myocardium and is mostly asymptomatic.13 The initial damage is thought to be caused by eosinophilic granules. The intermediate (thrombotic) phase is characterized by thrombus formation followed by organization of the thrombus into a thick layer of granulation tissue. In the third, fibrotic stage, granulation tissue changes into fibrosis, sometimes still with a small inflammatory zone.13 Nowadays the term ‘Löffler's endomyocarditis’ is used to describe the involvement of the heart in HES in the thrombotic and fibrotic stage.14 Typical echocardiographic findings in HES are endocardial thickening, fibrothrombotic obliteration of the ventricular apices, and valvular regurgitation due to restricted motion of the posterior mitral leaflet as assessed by 2D Doppler echocardiography.1–3,13
In the present study, most HES patients had no known cardiac disease or clinical signs of thrombosis or fibrosis at enrollment (except case 5). They were presumably in the first, asymptomatic stage, therefore the alterations in LA morphology and function would be related solely to HES. Only left ventricular hypertrophy and dilated LA could be detected by conventional 2D Doppler echocardiography, without significant valvular regurgitation or thrombus formation. 3DSTE confirmed LA volume changes in all phases of LA function and found alterations in both peak global and mean segmental LA CS, suggesting reduced reservoir function and LA remodeling.
A wide variety of pathophysiological changes may lead to LA remodeling, with structural, functional, neurohormonal or other consequences.15 The real mechanism behind LA remodeling in HES is not known, but myocyte necrosis and alterations in the extracellular matrix and in the release of atrial hormones due to toxic proteins from degranulating eosinophils and diastolic dysfunction could theoretically explain our findings.15
3DSTE is a new clinical tool for non-invasive 3D cardiac chamber quantifications of the left ventricle and atrium based on a block-matching algorithm of myocardial speckles during their frame-to-frame motion.4 3DSTE has been demonstrated to be useful for LA volumetric7–10 and strain10–12 assessments, allowing more detailed assessment of LA function from the same 3D dataset. Different patterns in 3DSTE-derived volume-based and strain functional properties can be demonstrated in different disorders. In a recent 3DSTE study, peak LA RS and LA LS were found to be altered in hypertrophic cardiomyopathy, together with preserved LA CS.10 In another study, strains at all LA levels showed alterations in atrial fibrillation by 3DSTE.12 In the present study, only peak LA CS was decreased in HES patients, while RS and LS remained unchanged. The real pathological mechanism behind the reason that only LA CS shows alterations in HES is not known, but hemodynamic reasons and their relationship with LA fiber orientation cannot be excluded, beyond the above-mentioned pathophysiologic factors.
LimitationsSeveral important limitations of the assessments should be taken into account, including lower image quality as compared to 2D echocardiography. Only a limited number of HES patients were examined, which should be considered when interpreting the results. However, HES is a relatively rare disease. Coronary angiography was performed in one HES case, with a negative result. Moreover, transesophageal echocardiography was not performed to exclude thrombi in HES patients.
ConclusionsIncreased LA volumes and stroke volumes were demonstrated in HES patients, accompanied by reduced LA peak circumferential strain as assessed by 3DSTE. These results suggest structural and functional LA remodeling in HES patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors express their thanks to Drs László Krenács and Enikő Bagdi for their opinion on the pathological aspects of the cases, and to Hajnalka Andrikovics for molecular tests.