There is disagreement whether white coat hypertensives (WCH) have different hemodynamic and structural characteristics compared to normotensives (NT) and hypertensives (HT).
MethodsWe compared cardiovascular prognostic markers (pulse wave velocity [PWV] and aortic stiffness index [ASI]) and data on central hemodynamics and central pressures (augmentation index [AIx], augmentation pressure [AugP] and pulse pressure amplification [PPA]) from aortic pulse wave analysis between NT (n=175), WCH (n=315) and treated HT (n=691), all with 24-h blood pressure (BP) <130/80 and nocturnal BP <120/70 mmHg after matching for age, gender, body mass index (BMI) and nocturnal BP. The groups were also compared separately in terms of 24-h systolic BP <120 mmHg and 120-129 mmHg.
ResultsThe percentage of non-dippers was 40.1% in NT, 34.5% in WCH and 38.3 in HT. For similar 24-h and nocturnal systolic BP (NT 109/64±7/5, WCH 110/66±7/6, HT 109/64±7/5 mmHg), aortic stiffness was greater in HT (n=691, PWV 10.8±2.6 m/s and ASI 0.33±0.16, p<0.01) than in WCH (n=316, PWV 9.7±2.4 m/s and ASI 0.28±0.17) and NT (n=175, PWV 9.5±2.0 m/s and ASI 0.29±0.15); AugP and AIx were higher (p<0.01) in HT (13.9±8.2 and 29.6±12.6 mmHg) than in WCH (11.5±8.5 mmHg and 24.9±15.2) and NT (11.0±6.4 mmHg and 26.6±11.5). PPA was lower (p<0.01) in HT (11.3±5.5 mmHg) than in WCH (13.2±7.1 mmHg) and in NT (12.4±4.9 mmHg). The findings were similar when the 24-h systolic BP <120 mmHg and 120-129 mmHg subgroups were analyzed separately.
ConclusionOur data suggest that for similar age, gender distribution, BMI, and 24-h and nocturnal BP, aortic stiffness, central aortic pressures and wave reflection in WCH are closer to those of NT than to those with treated HT. This supports the idea that white coat hypertension may be a more benign condition than treated hypertension for similar 24-h and particularly nocturnal BP levels.
Permanece controverso se os indivíduos com hipertensão da bata branca (HBBs) exibem alterações hemodinâmicas e estruturais diferentes dos indivíduos normotensos (NTs) e hipertensos (HTs).
MétodosComparamos marcadores de prognóstico cardiovascular (CV): velocidade da onda de pulso (VOP), índice rigidez aórtica (AASI) e as alterações da onda de pressão aórtica (índice de aumentação [AIx], pressão de aumentação [AugP] e amplificação central periférica da pressão de pulso [PPA]) entre NTs (n=175), HBBs (n=315) e HTs tratados (n=691) todos com pressão arterial (PA) de 24 h <130/80 e PA noturna <120/70 mm Hg, após emparelhamento para idade, género e IMC. Os grupos foram ainda comparados para PA 24 h <120 mm Hg e PA 24 h de 120-129 mm Hg.
ResultadosA percentagem de non dippers foi 40,1% nos NTs, 34,5% nos HBBs e 38,3% nos HTs. Para idêntica PA sistólica de 24 h e PA sistólica noturna (NT 109/64+7/5, HBB 110/66+7/6, HT 109/64+7/5 mm Hg), a rigidez aórtica foi mais elevada nos HTs (n=691, VOP=10,8+2,6 m/s e AASI 0,33+0,16, p<0,01) do que nos HBBs (n=316, PWV=9,7+2,4 m/s e AASI 0,28+0,17) e NTs (n=175, VOP=9,5+2,0 m/s e AASI 0,29+0,15); AugP e AIx foram mais elevadas (p<0,01) nos HTs (13,9+8,2 mm Hg e 29,6+12,6) que nos HBBs (11,5+8,5 mm Hg e 24,9+15,2) e NTs (11,0+6,4 mm Hg e 26,6+11,5). A PPA foi mais baixa p<0,01 nos HTs 11,3+5,5 do que nos HBBs 13,2+7,1 e do que nos NTs 12,4+4,9 mm Hg. Os dados foram semelhantes quando os subgrupos de PA 24 h <120 mm Hg ou entre 120-129 mm Hg foram analisados separadamente.
ConclusõesOs resultados sugerem que, para valores semelhantes da idade, IMC, género, PA ambulatória de 24 h e PA noturna, os HBBs apresentam valores da rigidez aórtica, da pressão central e das ondas refletidas mais próximos dos NTs do que dos HTs controlados. Estes dados permitem sugerir que a HBB constitui uma entidade relativamente benigna face à hipertensão sustentada para idênticos valores da PA de 24 h e, particularmente, da PA noturna.
It is accepted that 24-h ambulatory blood pressure (BP) monitoring (ABPM) is superior to office BP measurement in predicting cardiovascular risk.1 Individuals not taking antihypertensive medication who have persistently elevated office BP readings (>140 systolic and/or 90 mmHg diastolic) but normal BP otherwise have what is known as white coat hypertension (WCH).2 The European3 and American4 guidelines on hypertension state that a diagnosis of WCH requires such values to be observed on at least three occasion, together with average daytime or home measurements of <135/85 mmHg. Although these are the criteria3,5 most often used in studies, as shown in a recent meta-analysis,6 some authors suggest that confirmation of WCH requires two ABPM readings.
WCH, which appears to occur in at least 20% of the population with office hypertension,7,8 is the subject of heated debate with regard to its prognostic significance compared to normotension or sustained hypertension. Several studies9–15 have shown that WCH is associated with low prevalences of metabolic disturbances and target-organ damage compared to sustained hypertension, but other studies have documented more structural and functional abnormalities in target organs in white coat hypertensives than in normotensives.11,16–24 A greater tendency for individuals with WCH to go on to develop hypertension has been reported by some25 but not by others.26–28 In longitudinal studies, WCH was associated with lower mortality6 and a lower incidence of cardiovascular events than sustained hypertension,29–31 and in another study no difference was seen between WCH and normotension.30 By contrast, two other longitudinal studies32,33 found that the incidence of cardiovascular events in individuals with WCH was similar to those with hypertension and higher than in normotensives. However, in one of these studies32 many patients with WCH were diabetic and had been treated with antihypertensive medication, which does not fit the usual definition of WCH, while in the other,33 the older age of the WCH subjects compared to controls could explain their higher risk. These apparently contradictory findings may be due to differences in study populations and in the diagnostic criteria for WCH.
Two aspects of the question of the risk associated with WCH have yet to be addressed. One is that nocturnal BP values are not considered in the diagnosis of WCH. This could be important, since nocturnal BP has the highest predictive value for cardiovascular risk of all BP measures.1,34 Individuals with similar daytime BP levels may have different nocturnal BP levels, with important implications for cardiovascular risk. The second is that there may be differences between normotensives, white coat hypertensives and sustained hypertensives in terms of aortic pressure, central pressure wave and arterial wave reflection, independently of the office and ambulatory BP values used to diagnose WCH. Several studies35,36 have shown that increased pulse pressure (PP) amplification (PPA) and aortic stiffness, as well as early wave reflection, are associated with increased cardiovascular risk independently of peripheral BP.
The present study aimed to assess aortic stiffness, central pressure and arterial wave reflections in individuals with normotension, WCH and treated hypertension, matched for age, gender, body mass index (BMI) and 24-h and nocturnal BP.
MethodsThe study was approved by the ethics committee of Hospital Pedro Hispano. The population of this descriptive, cross-sectional, retrospective study consisted of all individuals registered in the databases of the Hypertension Unit of the Local Health Unit of Matosinhos who between 1999 and 2014 underwent 24-h ABPM and pulse wave velocity (PWV) measurement using the Complior device and analysis of central pressure curves using the SphygmoCor device. In 2014 the database included around 19000 ABPM records, 11000 PWV measurements and 6000 central pressure curves. The present study only includes records of individuals who at the time of assessment were aged over 18 years and had normal renal function (creatinemia <1.2 mg/dl), were not diabetic, and had no history of cardiovascular events.
Selection of study populationThe study subjects were classified at the time of ABPM and PWV measurement as normotensive, white coat hypertensive, or hypertensive. Normotensives were recruited over the years from hospital staff and their relatives, and were defined as those with office BP <140/90 mmHg on three occasions; they had undergone clinical assessment which showed them to be healthy, with no known disease and no history of cardiovascular medication, and daytime BP of <135/85 mmHg on voluntary ABPM. Individuals with office systolic BP (SBP) of >140 mmHg or diastolic BP >90 mmHg measured on three occasions, not under antihypertensive therapy and with daytime BP of <135/85 mmHg on ABPM, were considered to have WCH, and those with a previous diagnosis of essential hypertension and under antihypertensive therapy for at least six months at the time of ABPM were considered to be hypertensive. Only individuals with mean 24-h BP of <130/80 mmHg and nocturnal BP of <120/70 mmHg on ABPM were included, which means that among hypertensives, only those whose hypertension was adequately controlled were selected.
Selected individuals were then randomized using a four-way table for stratification and pairing of the different groups. Subjects’ data were entered into the table in the proportion 1:2:4, so that for every normotensive individual there were two white coat hypertensives and four hypertensives. The total population was divided into two groups: those with 24-h SBP <120 mmHg and those with 24-h SBP 120-129 mmHg. Both these groups were then stratified by BP group (normotensive, white coat hypertensive, or hypertensive), gender (male or female), age (25-40 years, 41-65 years, or 66-80 years), and BMI (20-24.9 kg/m2, 25-29.9 kg/m2, or 30-35 kg/m2). The final study population consisted of 175 normotensives, 315 white coat hypertensives and 691 hypertensives. The differences in these numbers are due to the disproportion between these groups in the database (more hypertensives than white coat hypertensives or normotensives). The pairing method used was designed to minimize the selection bias that would have arisen if the sample sizes of white coat hypertensives and hypertensives had been the same as that of the smallest stratum (normotensives).
Office and 24-h ambulatory blood pressureOffice BP was measured in accordance with the World Health Organization guidelines, using a validated device (Omron HEM 705CP, Omron Inc., Vernon Hills, IL). Measurements were taken with the subject seated and the mean of three readings were used for the present study. All subjects underwent 24-h ABPM using a SpaceLabs 90207 on a normal working day, with readings taken every 20 min during the day and every 30 min at night (the division between daytime and nocturnal periods was based on the patient's diary entries for rising and going to bed). Only records with >85% of valid measurements were used. On the basis of each 24-h record, heart rate, mean 24-h, daytime and nocturnal BP, and percentage nocturnal BP fall were analyzed, as described in other publications by our group.37–39
Aortic stiffness indexThe aortic stiffness index (ASI) is derived from ABPM values and is calculated by (1−[slope of diastolic versus systolic blood pressure]).40,41 It is considered to have prognostic value.40,41
Pulse wave velocityCarotid-femoral PWV, an index of aortic distensibility, was determined in all subjects using the automated Complior system (Colson, Garges-lès-Gonesse, France), as described previously.13,27,37–39,42,43
Central pressure waveThe aortic pressure curve was derived, using a previously validated method, from radial and carotid BP waves measured directly by applanation tonometry using the SphygmoCor device (Atcor Medical, Sydney, Australia), as previously described.37–39 As well as peripheral (brachial) PP, the following parameters were derived from the aortic pressure wave after calibration with brachial BP: SBP, central PP (PPc), BP at the augmentation point (Pinc at the first incisura of the systolic phase of the curve), augmentation pressure (AugP) added to Pinc, attributed to the projection of reflected pressure waves on the BP curve. We also calculated the duration (ΔTp) of projection of reflected waves (from beginning of systole to the augmentation point), total left ventricular ejection duration and the augmentation index (AIx, in %) adjusted for a heart rate of 75 bpm, calculated by the AugP/PP ratio in the aortic pressure curve, as an indicator of the effect of wave reflections on the central arteries.
Statistical analysisThe results are expressed as means ± standard deviation. Data were compared between groups mainly by analysis of variance for repeated measures, followed by post-hoc analysis of multiple comparisons using the Tukey test to determine the significance of differences between any two groups. A value of p<0.05 was considered statistically significant.
ResultsAfter selection and stratification, the final study population consisted of 175 normotensives, 315 white coat hypertensives and 691 hypertensives. Table 1 shows the characteristics of the three groups. There were no significant differences between the groups in terms of age, gender, BMI, low-density lipoprotein cholesterol (LDL-C), or 24-h or nocturnal BP. As expected, normotensives presented lower office BP levels than the other groups, while hypertensives had higher values in the two indices of arterial stiffness (PWV and ASI), which did not differ between normotensives and white coat hypertensives. Regarding parameters of central pressure, compared to normotensives and white coat hypertensives, hypertensives had higher PPc, AIx and AugP and lower PPA, and included a greater percentage with microalbuminuria. There were no significant differences in any of these parameters between normotensives and white coat hypertensives.
Characteristics of the study population and values of office and ambulatory blood pressure and pulse wave velocity in normotensives, white coat hypertensives and hypertensives.
| NT | WCH | HT | p | |
|---|---|---|---|---|
| n | 175 | 316 | 691 | |
| Age (years) | 48±13 | 48±15 | 50±17 | NS |
| Female (%) | 54 | 55 | 55 | NS |
| BMI (kg/m2) | 27±5 | 27±4 | 28±5 | NS |
| Office SBP (mmHg) | 125±9* | 146±12 | 143±16 | <0.001 |
| Office DBP (mmHg) | 79±7* | 91±10 | 87±12 | <0.001 |
| 24-h SBP (mmHg) | 119±6 | 120±5 | 120±7 | NS |
| 24-h DBP (mmHg) | 71±5 | 73±7 | 72±7 | NS |
| 24-h HR (bpm) | 73±8 | 74±10 | 72±10 | NS |
| Nocturnal SBP (mmHg) | 109±7 | 110±7 | 109±7 | NS |
| Nocturnal DBP (mmHg) | 64±5 | 66±6 | 64±5 | NS |
| Non-dippers (%) | 40.1 | 34.5 | 38.3 | NS |
| PPc (mmHg) | 12.4±4.9 | 13.2±7.1 | 11.3±5.5* | <0.01 |
| AIx 75 (%) | 26.6±11.5 | 24.9±15.2 | 29.6±12.6 | <0.01 |
| PPA (mmHg) | 11.0±6.4 | 11.5±8.5 | 13.9±8.2* | <0.01 |
| PWV (m/s) | 9.5±2.0 | 9.7±2.4 | 10.8±2.6* | <0.01 |
| ASI | 0.29±0.15 | 0.28±0.17 | 0.33±0.16* | <0.01 |
| Blood glucose (mg/dl) | 92±18 | 95±21 | 99±27 | NS |
| eGFR (ml/min/1.73 m2) | 95±24 | 93±21 | 88±25 | NS |
| LDL-C (mg/dl) | 123±35 | 125±36 | 125±36 | NS |
| Microalbuminuria (%) | 7 | 11 | 19* | 0.04 |
AIx 75: augmentation index adjusted for heart rate of 75 bpm; BMI: body mass index; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate by the Modification of Diet in Renal Disease (MDRD) formula; HR: heart rate; HT: hypertension; LDL-C: low-density lipoprotein cholesterol; microalbuminuria: 24-hour urinary albumin excretion >29 mg; NT: normotension; PPA: pulse pressure amplification; PPc: central pulse pressure; PWV: pulse wave velocity; SBP: systolic blood pressure; WCH: white coat hypertension.
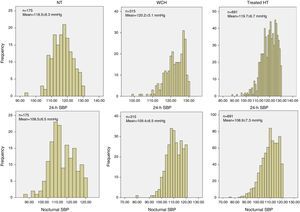
Figure 1 shows the distribution of 24-h and nocturnal SBP levels in the three groups. As a result of the selection criteria used, a gaussian distribution can be seen in the hypertensive group but not in the other two groups, even though the mean values of the three groups are not significantly different.
Distribution of 24-h and nocturnal systolic blood pressure levels in normotensives (n=175), white coat hypertensives (n=316), and treated hypertensives (n=691), matched for age, gender, body mass index and 24-h and nocturnal blood pressure. HT: hypertensives; NT: normotensives; SBP: systolic blood pressure; WCH: white coat hypertensives.
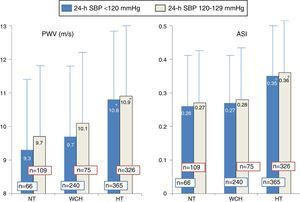
Figure 2 shows PWV and ASI values in the three groups, divided according to 24-h SBP: low (<120 mmHg) or high (120-129 mmHg). Higher values in hypertensives than in normotensives and white coat hypertensives were observed both for individuals with 24-h SBP <120 mmHg and with 120-129 mmHg, as also shown in Table 1 in the overall population.
Pulse wave velocity and aortic stiffness index in normotensives (n=175), white coat hypertensives (n=316), and treated hypertensives (n=691), according to 24-h systolic blood pressure <120 mmHg or 120-129 mmHg. The number of individuals in each group is indicated on the corresponding column. ASI: aortic stiffness index; HT: hypertensives; NT: normotensives; PWV: pulse wave velocity; SBP: systolic blood pressure; WCH: white coat hypertensives.
*p<0.04 (significantly different from normotensives and white coat hypertensives in the same group).
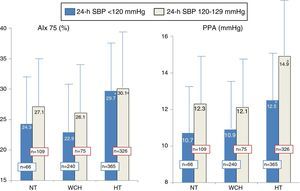
Figure 3 presents AIx and PPA values in the three groups according to 24-h SBP: low (<120 mmHg) or high (120-129 mmHg). As in Figure 2, values were higher in hypertensives than in normotensives and white coat hypertensives both for individuals with 24-h SBP <120 mmHg and with 120-129 mmHg, as was also the case in the overall population.
Values of augmentation index and pulse pressure amplification calculated from the central pressure wave in normotensives (n=175), white coat hypertensives (n=316), and treated hypertensives (n=691), according to 24-h systolic blood pressure <120 mmHg or 120-129 mmHg. The number of individuals in each group is indicated on the corresponding column. AIx 75: augmentation index adjusted for heart rate of 75 bpm; HT: hypertensives; NT: normotensives; SBP: systolic blood pressure; WCH: white coat hypertensives.
*p<0.04 (significantly different from normotensives and white coat hypertensives in the same subgroup).
The present study assessed central pressures and aortic stiffness in a population of white coat hypertensives compared to normotensives and controlled hypertensives. The groups were carefully matched for age, gender, BMI and ambulatory BP values.
Indices of aortic stiffness (PWV and ASI) and central BP and peripheral wave reflection in white coat hypertensives did not differ significantly from those of normotensives but were considerably lower than in hypertensives. WCH is a common condition, with a prevalence of over 20% of the population diagnosed as hypertensive based on office BP.7,8 However, the findings of clinical trials and meta-analyses have fueled a heated debate as to whether WCH has a benign prognosis6,9–15,26–29,31 or is associated with increased cardiovascular risk16–20,22–25,32,33 compared to normotension and sustained hypertension. Since meta-analyses34,44 that include most studies comparing ABPM with office BP demonstrate that ABPM has higher prognostic value than office BP, WCH, in which ABPM values are normal, should in theory have a relatively benign prognosis.
The reasons for the conflicting results include differences in study populations with WCH and the influence of the white coat effect and cardiovascular risk factors such as age, gender, BMI, and diabetes.45,46. Thus, in order to minimize the effect of these variables in our study, we took great care to match white coat hypertensives, normotensives and hypertensives for age, gender, BMI and ambulatory BP. Furthermore, LDL-C values did not differ between the three groups, although we cannot exclude the possibility that this was due to different rates of use of lipid-lowering drugs.
To our knowledge, only one study46 has assessed arterial wave reflections in white coat hypertensives, in which factors influencing WCH were not as rigorously controlled. In our study hypertensives, even though well controlled, presented higher indices of aortic stiffness (PWV and ASI) than white coat hypertensives and normotensives. Similarly, indices derived from the central pressure wave (PPc, PPA and AIx) were less favorable in hypertensives than in the other two groups, both in the overall population and in the 24-h systolic BP <120 mmHg and 120-129 mmHg subgroups.
Although values for indices of aortic stiffness and central hemodynamics were slightly higher in white coat hypertensives than in normotensives in our study, this tendency did not reach statistical significance. Various studies have shown that increased PP and aortic stiffness and early wave reflection are independently associated with higher cardiovascular risk.35,36 Greater aortic stiffness increases BP, PP and PWV, which in turn causes peripheral wave reflection to occur early, in systole rather than in diastole.47,48 Early wave reflection, as typically seen in aging and in hypertension, leads to increased PP in the central more than the peripheral arteries, disrupting the normal amplification of PP between the aorta and the peripheral vasculature.
Our findings suggest that white coat hypertensives have a more favorable profile in terms of aortic distensibility and central pressures than hypertensives. White coat hypertensives and normotensives had similar hemodynamics and vascular structure, with similar effects on central pressure. Lower indices of aortic stiffness (lower PWV and ASI) and lower PPc for the same brachial BP gave these two groups more favorable PPA values than in the hypertensive group.
Aortic pulse wave analysis by applanation tonometry in this study showed that normotensives and white coat hypertensives presented lower AIx and AugP than hypertensives, which suggests that wave reflections from peripheral arteries are weaker in WCH. Several studies have suggested that PPc depends, among other factors, on the strength and timing of peripheral wave reflection and duration of ventricular ejection, and that reducing pulse wave reflection could lower central SBP without significantly altering peripheral pressures.49
The most important finding of our study was that aortic stiffness and central hemodynamics in white coat hypertensives are closer to those of normotensives than to those of hypertensives, for similar age, gender distribution, BMI, and ambulatory daytime and nocturnal BP. These data contrast with those of other studies in which increased aortic stiffness was seen in WCH, either intermediate between normotensives and hypertensives51 or similar to those with masked hypertension.52 However, it should be noted that these studies did not match the groups as rigorously for variables such as age, comorbidities or daytime BP on ABPM, and the diagnostic criteria used for WCH were not standard, since some patients were under therapy.
Almost all studies assessing WCH have used only daytime BP values (<135/85 mmHg), as recommended in the international guidelines.3,5 To the best of our knowledge, this is the first study in which normal nocturnal BP (<120/70 mmHg) was a diagnostic criterion for the diagnosis of WCH. Since nocturnal BP is known to be a better predictor of cardiovascular risk,5,34,44,50 this requirement may have had the effect of making WCH appear a more benign condition.
Indices of arterial stiffness and central pressure are important markers of target organ damage and predictors of cardiovascular risk,35,36,41 and the fact that these indices were relatively normal in white coat hypertensives indicates that WCH is a benign condition, although we cannot exclude the possibility that it may confer some additional cardiovascular risk. These findings support the guidelines,3–5 which recommend vigilance but not medication, since there is insufficient evidence that it is of benefit.
LimitationsOur study has certain limitations. The first is that, as in all cross-sectional studies, causal relationships are difficult to establish. The second is that the particularly rigorous matching process may have led to the selection of a population at relatively low risk, and thus not fully representative of all white coat hypertensives.
ConclusionOur data suggest that for similar age, gender distribution, BMI, and 24-h and nocturnal BP, aortic stiffness, central pressures and wave reflection in white coat hypertensives are closer to those of normotensives than to treated hypertensives. This means that for those with normal 24-h and nocturnal BP, WCH may be a relatively benign condition compared to hypertension, although the possibility cannot be excluded that it may confer some additional cardiovascular risk compared to normotension.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Dr. Maria Helena Silva for her linguistic revision of the text.
Please cite this article as: Almeida J, Monteiro J, Silva JA, et al. Os valores da pressão arterial aórtica e índice de aumentação central em indivíduos com hipertensão da bata branca são mais próximos dos indivíduos normotensos do que dos hipertensos tratados para idênticas idades, género e pressão noturna. Rev Port Cardiol. 2016;35:559–567.