To combine the results of the best scientific evidence in order to compare the effects of cardiac resynchronization therapy (CRT) in heart failure patients with atrial fibrillation (AF) and in sinus rhythm (SR) and to determine the effect of atrioventricular nodal ablation in AF patients.
MethodsThe electronic databases PubMed, B-On and Cochrane CENTRAL were searched, and manual searches were performed, for randomized controlled trials and cohort studies up to November 2012. The endpoints analyzed were all-cause and cardiovascular mortality and response to CRT.
ResultsWe included 19 studies involving 5324 patients: 1399 in AF and 3925 in SR. All-cause mortality was more likely in patients with AF compared to patients in SR (OR=1.69; 95% CI: 1.20–2.37; p=0.002). There were no statistically significant differences in cardiovascular mortality (OR=1.36; 95% CI: 0.92–2.01; p=0.12). AF was associated with an increased likelihood of lack of response to CRT (OR=1.41; 95% CI: 1.15–1.73; p=0.001). Among subjects with AF, ablation of the atrioventricular node was associated with a reduction in all-cause mortality (OR=0.42; 95% CI: 0.22–0.80; p=0.008), cardiovascular death (OR=0.39; 95% CI: 0.20–0.75; p=0.005) and the number of non-responders to CRT (OR=0.30; 95% CI: 0.10–0.90; p=0.03).
ConclusionsThe presence of AF is associated with increased likelihood of all-cause death and non-response to CRT, compared to patients in SR. However, many patients with AF benefit from CRT. Atrioventricular nodal ablation appears to increase the benefits of CRT in patients with AF.
Combinar os resultados da melhor evidência científica de forma a comparar os efeitos da terapêutica de ressincronização cardíaca (TRC) em doentes com insuficiência cardíaca em fibrilhação auricular (FA) e em ritmo sinusal (RS) e determinar a influência da ablação auriculoventricular (AV) no grupo de doentes em FA.
MétodosA pesquisa realizou-se nas bases de dados eletrónicas da PubMed, B-On e CENTRAL e de forma manual, incluindo ensaios clínicos controlados aleatorizados e estudos de coorte até novembro de 2012. Analisou-se a mortalidade total e cardiovascular e a resposta à TRC.
ResultadosForam incluídos 19 estudos que envolveram 5324 pacientes: 1399 em FA e 3925 em RS. O grupo com doentes em FA apresenta maior risco de mortalidade total, comparativamente ao grupo de doentes em RS (OR=1,69; IC 1,20–2,37, p=0,002). Não foram verificadas diferenças estatisticamente significativas quanto à mortalidade cardiovascular (OR=1,36, IC 0,92–2,01, p=0,12). A não resposta à TRC foi maior no grupo em FA (OR=1,41; IC 1,15–1,73; p=0,001). Entre os indivíduos em FA, a ablação do nódulo auriculoventricular foi associada à redução da mortalidade total (OR=0,42; IC 0,22–0,80; p=0,008), mortalidade cardiovascular (OR=0,39; IC 0,20–0,75; p=0,005) e número de não respondedores à TRC (OR=0,30; IC 0,10–0,90; p=0,03).
ConclusõesA presença de FA está associada a maior probabilidade de morte por todas as causas e de não resposta à TRC, comparativamente aos doentes em RS. Contudo, um número significativo de doentes em FA beneficia da TRC. A ablação AV parece aumentar os benefícios da TRC nos doentes com FA.
Atrial fibrillation (AF) is the most common arrhythmia in patients with heart failure (HF) and is associated with increased mortality and morbidity.1 About 20% of patients treated with cardiac resynchronization therapy (CRT) are in AF.2,3 Despite the high prevalence of AF in patients with HF and the fact that many meet the criteria for CRT, randomized controlled trials have excluded these patients in most cases.4 Thus, the effect of this therapy in patients with AF is still controversial.
Notwithstanding this controversy, according to the American Heart Association, American College of Cardiology and Heart Rhythm Society guidelines, CRT is a class IIa recommendation (level of evidence B) for patients with AF, left ventricular ejection fraction (LVEF) ≤35% and ventricular dyssynchrony, since a high percentage of biventricular capture can be ensured. Atrioventricular (AV) nodal ablation should be performed in cases of incomplete biventricular capture.5
AV nodal ablation offers the most effective method for rate control in AF patients, by creating a complete heart block and regularizing cardiac rhythm through permanent pacing. This approach enables complete biventricular pacing.6 Nevertheless, the importance of AV nodal ablation (compared to pharmacologic therapy) in achieving an optimized response to CRT in AF patients remains unclear.
The meta-analyses published by Upadhyay et al. in 20087 and by Wilton et al. in 20118 suggested significant differences in outcomes between patients in SR and those in AF, highlighting the need for further research.6
The aim of this meta-analysis was to investigate the effects of CRT in patients with AF compared with patients in SR, and to evaluate the effect of AV nodal ablation in the former group. To the best of our knowledge, this is the most recent and up-to-date meta-analysis on this subject.
MethodsSearch strategySearches were conducted in the electronic databases PubMed, B-On, and Cochrane Central Register of Controlled Trials, and included the following terms: “atrial fibrillation”, “heart failure”, “congestive heart failure”, “congestive cardiac failure”, “chronic heart failure”, “chronic cardiac failure”, “resynchronization therapy”, “cardiac resynchronization therapy”, “cardiac resynchronization”, “heart resynchronization”, “artificial biventricular pacemaker”, “biventricular pacemaker”, “biventricular pacing”, “biv”, “dual-chamber pacing”, “dual-chamber pacemaker”, “atrioventricular nodal ablation”, “atrioventricular junction ablation”, “ablation pacing”, “ablation techniques”, “ablation”, “AV nodal ablation”, “AVJ ablation”.
We considered studies in humans, published and unpublished, written in English or Portuguese, up to November 2012. In addition, we performed a manual search of abstracts in journals and conferences and in reference lists of selected articles.
Inclusion criteriaWe included studies that met all of the following criteria: randomized controlled trials or cohort studies; individuals with a diagnosis of HF in NYHA class II–IV with LVEF ≤35%, in AF or SR; comparison between patients in AF and SR; implantation of cardiac resynchronization devices; study of all-cause mortality, cardiovascular mortality, non-responders to therapy and follow-up ≥6 months.
Data extractionFrom each study, the following data were extracted and analyzed: characteristics of the study population, study design, methodological criteria, interventions, outcomes of interest and results. All text, tables and figures were reviewed for data extraction.
The primary endpoint was all-cause mortality. The secondary endpoints included cardiovascular mortality and non-response to CRT. If the investigators reported endpoints at two different follow-up times, endpoints for the longest available duration of follow-up were used.
Quality assessmentAssessment of studies’ quality and eligibility and their selection were performed independently by two investigators. Studies that did not meet the inclusion criteria were excluded from the meta-analysis. Disagreements were resolved by consensus. We included articles by the same author(s) if they had different samples or analyzed different outcomes.
The quality of included studies was assessed using a checklist developed by the reviewers. This instrument analyzed a series of items involving methodology, participants, interventions and outcomes, designed to minimize bias.
Statistical analysisThe statistical analysis was performed with Review Manager (RevMan) statistical software, version 5.1.
For dichotomous clinical outcomes, overall estimation of the treatment effect was performed with the Peto statistical method, through a random effects model. Odds ratios (OR) and corresponding 95% confidence intervals were calculated, and the Z-test was used to determine the statistical significance of the estimated overall effect. Statistical heterogeneity was quantified using Cochran's chi-square test. The I2 statistic was also calculated to assess the inter-study consistency of the results. An I2 of 25%, 50% and 75% indicates low, moderate or high heterogeneity, respectively. Sensitivity analysis was performed to identify reasons for heterogeneity. Different strategies included changing criteria for inclusion of studies according to their methodological characteristics; exclusion of studies that showed some ambiguity in their inclusion criteria; exclusion of unpublished studies; and re-evaluation of the data using different statistical methods, such as calculation of risk ratios (RR) instead of OR, calculated by both fixed and random effect models. These multivariate analyses were performed to determine whether modification of some criteria would be sufficient to change the combined result and thus assess the degree of confidence of the results of the meta-analysis.
A funnel plot was used to assess publication bias. This analysis identifies inter-study asymmetries that should be explored. In the absence of bias, dispersion of the points of the plot is similar to a symmetric pyramid. The presence of asymmetry in the graph suggests the existence of publication bias.
A p-value of <0.05 was adopted as the criterion for statistical significance.
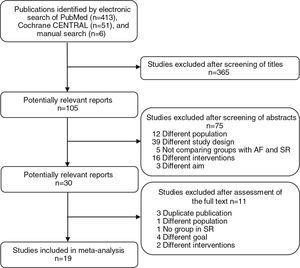
ResultsSearch resultsThe search retrieved 470 potentially relevant articles. The first stage of selection, based on titles, excluded 365 articles. Of the 105 studies considered, 75 were excluded after examination of the abstract. The full text of the remaining 30 publications was examined in more detail. Finally, 19 articles were included in our meta-analysis (Figure 1).
Description of included studiesTable 1 summarizes the characteristics of the included articles, which involved a total of 5325 patients (1399 with AF and 3925 in SR). Mean age was 67.7 years in the AF patients and 66.2 years in the SR patients. Comparing patients with and without AF, the proportion of males was 81.3% vs. 75.1%, ischemic cardiomyopathy was present in 40.6% vs. 45.5%, LVEF was 24.5% vs. 24.3%, and QRS duration was 171.8 ms vs. 167.9 ms, respectively. Most participants reported symptoms of advanced HF (NYHA functional class III and IV). Length of follow-up ranged from 6 to 34 months.
Characteristics of the included studies.
| Study | Type | Patients (n) | Age (years) | Men (%) | Follow-up (months) | CRT-D (n) | |||||
| AF | SR | AF | SR | AF | SR | AF | SR | AF | SR | ||
| Leclercq at al.35 | Prosp | 15 | 22 | 68±6 | 67±8 | – | – | 14 | 14 | – | – |
| Linde et al.37 | RCT | 64 | 67 | 65±9 | 63±10 | 81 | 75 | 12 | 12 | – | – |
| Molhoek et al.9 | Prosp | 30 | 30 | 63±10 | 68±8 | 90 | 80 | 19 | 25 | 13 | 15 |
| Gasparini et al.40 | Prosp | 162 | 511 | 66±8 | 63±10 | 86 | 77 | 25 | 26 | 79 | 299 |
| Delnoy et al.18 | Prosp | 96 | 167 | 73±8 | 72±9 | 75 | 68 | 23 | 23 | – | – |
| Buck et al.12 | Prosp | 56 | 58 | 63±11 | 62±12 | 70 | 79 | 18 | 18 | – | – |
| Ferreira et al.10 | Retrosp | 53 | 78 | 69±9 | 66±10 | 94 | 74 | 29 | 29 | 43 | 59 |
| Gasparini et al.4 | Retrosp | 243 | 42 | 66±9 | 63±10 | 82 | 75 | 34 | 34 | – | – |
| Khadjooi et al.43 | Prosp | 86 | 209 | 72±10 | 68±11 | 86 | 77 | 24 | 22 | – | – |
| Tolosana et al.15 | Retrosp | 126 | 34 | 69±7 | 67±9 | 81 | 76 | 12 | 12 | 65 | 215 |
| Schütte et al.44 | Retrosp | 36 | 64 | – | – | – | – | 11 | 11 | – | – |
| Kim et al.13 | – | 26 | 96 | – | – | – | – | 6 | 6 | – | – |
| Wo et al.11 | Retrosp | 16 | 40 | 68±13 | 66±14 | 69 | 67 | 6 | 6 | – | – |
| Luedorff et al.16 | Retrosp | 139 | 445 | 71±7 | 68±9 | 81 | 71 | 24 | 24 | – | – |
| Wilton et al.17 | Retrosp | 19 | 67 | 68±7 | 69±12 | 89 | 88 | 34 | 34 | – | – |
| Tolosana et al.14 | Retrosp | 46 | 156 | 68±9 | 66±9 | 71 | 77 | 12 | 12 | 33 | 136 |
| Himmel et al.45 | Prosp | 46 | 230 | 69±9 | 70±8 | – | – | 12 | 12 | – | – |
Data expressed as mean ± SD. AF: atrial fibrillation; CRT-D: cardiac resynchronization therapy with defibrillator; Prosp: prospective; RCT: randomized controlled trial; Retrosp: retrospective; SR: sinus rhythm.
Diuretics were prescribed in 90% vs. 85% of patients, beta-blockers in 66% vs. 70%, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers in 94% vs. 90%, amiodarone in 35% vs. 22% and digitalis in 56% vs. 39% in the AF and SR groups, respectively.
There were statistically significant differences in baseline characteristics between patients with AF and SR in most studies included.
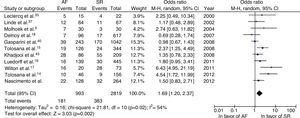
All-cause mortalityWhen the results of the 11 randomized trials were pooled, the odds ratio for overall mortality was 1.69 (95% confidence interval [CI]: 1.20–2.37; p=0.002), meaning a 69% higher probability of death in patients with AF compared with patients in SR. We found statistical evidence of moderate heterogeneity (I2=54%; p=0.02) (Figure 2).
We explored potential causes of heterogeneity between studies through sensitivity analyses. After exclusion of an unpublished study (Nascimento C, Pereira T, Providência R, Rodrigues P), there was no change in the pooled estimate using a random effects model (OR: 1.74; 95% CI: 1.18–2.57; p=0.005; I2=59%). The inclusion of six studies in which no individuals underwent AV nodal ablation resulted in a total effect of 1.58 (95% CI: 0.97–2.56; p=0.06), with heterogeneity of 47%, compared to an OR of 1.87 (95% CI: 1.08–3.23; p=0.02) and heterogeneity of 68% in the other five studies.
Excluding studies that included patients in NYHA class II, the increase in all-cause mortality in AF patients was even more pronounced (OR: 2.14; 95% CI: 1.47–3.09; p<0.0001; I2=32%). In the sensitivity analysis that included only cohort studies, a similar increase in all-cause mortality was observed (OR: 1.76; 95% CI: 1.22–2.54; p=0.003; I2=58%).
In order to explore statistical heterogeneity and investigate potential publication bias, a funnel plot was constructed, which revealed qualitative evidence of asymmetry in the distribution of the estimated effects in several studies. The analysis of all-cause mortality was redone excluding studies with greater dispersion. This produced an OR of 1.38 (95% CI: 1.07–1.78), a statistically significant difference (p=0.01), and a decrease in heterogeneity (I2=20%).
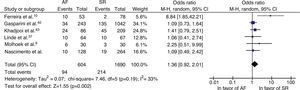
Cardiovascular mortalityPooled analysis of six studies comparing cardiovascular mortality in patients with AF and SR after treatment with CRT showed no statistically significant differences between groups (OR: 1.36; 95% CI: 0.92–2.01; p=0.12), although there was a trend in favor of the SR group. There was mild heterogeneity (I2=33%; p=0.19) (Figure 3).
Non-response to cardiac resynchronization therapyResponse to CRT was defined as improvement of one NYHA functional class after six months,9,10 decrease in left ventricular end-systolic volume (LVESV) of 10% or 15% measured by cardiac ultrasound,2,11–13 or improvement in absolute LVEF of ≥5% after three months.13 In studies by Tolosana et al.,14,15 clinical responders were defined as those with an increase of ≥10% in the 6-minute walk test 12 months after implantation or improvement of at least one NYHA functional class. Echocardiographic responders were defined as patients who had a ≥10% reduction in LVESV.14 Other trials used a definition of CRT response that required both objective symptomatic improvement in quality of life and a ≥15% reduction in LVESV.8,13
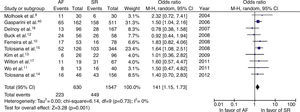
The rate of non-response to CRT was significantly higher in individuals with AF (OR=1.41, 95% CI: 1.15–1.73, p=0.001). No statistical heterogeneity was found (I2=0%) (Figure 4). The overall mean proportion of responders was 65% in those with AF and 71% in those in SR. Excluding the three studies that included patients with paroxysmal AF gave similar results (OR=1.56, 95% CI: 1.23–1.99, p=0.0002, I2=0%).
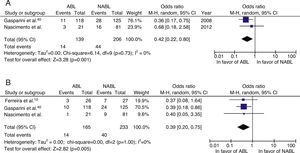
The role of atrioventricular nodal ablationWe identified two studies that evaluated the impact of ablation on all-cause mortality in AF patients. In these studies, mortality was 58% (OR=0.42) lower in the group who underwent AV nodal ablation. Pooled analysis of three studies showed that cardiovascular mortality was 61% (OR=0.39) lower in this group than in those who did not undergo AV nodal ablation. In both analyses, the overall effect was statistically significant (p=0.008 and p=0.005, respectively), with no evidence of heterogeneity between studies (I2=0%) (Figure 5).
Meta-analysis comparing all-cause mortality (A) and cardiovascular mortality (B) in patients with atrial fibrillation who did or did not undergo atrioventricular nodal ablation. ABL: atrioventricular nodal ablation; AF: atrial fibrillation; NABL: no atrioventricular nodal ablation (pharmacological therapy); SR: sinus rhythm.
In four studies analyzed, the likelihood of non-response to CRT was 69% lower in patients treated by ablation (OR: 0.31; 95% CI: 0.10–0.97; p=0.04; I2=72%). There was moderate inconsistency, which means the results should be treated with caution.
DiscussionThis meta-analysis suggests that AF, compared to SR, is associated with increased all-cause mortality in patients undergoing CRT. This finding was particularly robust when re-evaluated in sensitivity analyses performed to explore heterogeneity. Furthermore, although cardiovascular mortality was not significantly different between groups, there was a trend in favor of SR, and the large standard deviation in some studies may have reduced the statistical significance of their results. Therefore, we cannot exclude small differences in cardiovascular mortality between patients with AF and SR. Finally, our meta-analysis suggests that AV nodal ablation decreases all-cause and cardiovascular mortality in AF patients. To the best of our knowledge, this is the most recent and up-to-date meta-analysis on this subject, which increases its clinical applicability.
Our findings are consistent with those of other studies.9,14,16,17 In a systematic review and meta-analysis of 23 studies, Wilton et al. suggested that AF was associated with a weaker clinical response to CRT and an increased risk of death from any cause.8 By contrast, Molhoek et al.9 and Delnoy et al.18 found no differences in survival between subjects with AF and SR. Some investigators have suggested that digoxin and amiodarone may increase morbidity and mortality in patients with HF.19–21 Some studies included in this meta-analysis showed significant differences in medication between the AF and SR groups, with more patients in the former treated with these drugs. This difference may have contributed to a worsening of all-cause mortality in these patients.
The Resynchronization for Ambulatory Heart Failure Trial (RAFT) randomized patients to an implantable cardioverter-defibrillator (ICD) and cardiac resynchronization therapy with defibrillator (CRT-D) in patients with permanent AF and found no differences in cardiovascular mortality, heart failure hospitalization, 6-minute walk test or rate of perioperative complications.22 Our meta-analysis contradicts these findings to some extent, as a clear benefit from CRT was shown in the AF group.
Patients with AF had a higher rate of lack of response to CRT. The rates of non-response in the two groups were similar to those of previous studies,9,11,23,24 despite the fact that the definition of response to CRT differs widely in the literature.25 In their 2011 meta-analysis of 23 studies, Wilton et al. reported higher rates of non-response to CRT in patients with AF and a clinical benefit from AV nodal ablation in this group,8 which is corroborated by our findings. In fact, our meta-analysis strongly suggests the ablation procedure may be associated with increased survival and response rate to CRT in subjects with AF.
Considering that AF in patients with HF is associated with worse prognosis and higher mortality,26 a higher adverse event rate and a lower probability of response to CRT would be expected in these patients. In this context, the benefits found in patients with this arrhythmia, although less than those reported in patients in SR, are clinically significant and may be proportional; in other words, CRT could have the same impact in patients in SR and AF when adjusted for the overall greater comorbidity and frailty of the latter group. On the other hand, the possibility that AF impacts directly on the outcome of CRT should be considered, as AF has already been unequivocally demonstrated to predict mortality in patients with structural cardiac abnormalities and HF.15,27–30 Our results suggest that CRT benefits patients with AF, even if less than those in SR.
AF can directly impair the function of CRT devices by reducing biventricular capture, due to loss of AV synchrony and rapid and irregular ventricular rate, which has been associated with adverse outcomes.31,32 Therefore, AV junction ablation may be of particular value, as it ensures adequate biventricular pacing in patients with AF. Nevertheless, potential benefits must be balanced against the risks associated with pacemaker dependency. Observational studies have investigated the effects of AV nodal ablation in patients with HF and AF treated with CRT and demonstrated benefits in left ventricular systolic function, NYHA class, mitral regurgitation and exercise capacity.9,10,33–38 A study by Brignole et al. included 186 patients randomized to CRT or conventional right ventricular (RV) pacing following AV junction ablation. These investigators proposed that CRT is superior to RV apical pacing in reducing the clinical manifestations of HF in patients with AF, providing further evidence that CRT is effective in patients with AF, especially if AV junction ablation is performed concurrently.39 Gasparini et al. found that patients with AF who underwent AV nodal ablation showed significant improvement in LVEF, NYHA class and exercise capacity, a better response to CRT and faster reverse remodeling. All-cause mortality was significantly lower in patients with AV nodal ablation compared with those treated pharmacologically. These data, together with the findings of our meta-analysis, suggest that patients with AF can benefit from ablation and pacing.4,40 AV nodal ablation ensures 100% capture and heart rate control, which are difficult to achieve with pharmacological treatment, overcoming the deleterious effects of rapid and irregular ventricular rate and competitive pacing.41 In fact, in the 2013 ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR Appropriate Use Criteria, CRT was deemed appropriate to use in any situation where >40% RV pacing is anticipated, such as after AV junction ablation.42 Moreover, AV nodal ablation enables discontinuation of drugs such as digoxin and amiodarone, which can have a positive impact on mortality and morbidity in patients with HF, as mentioned above.
Notwithstanding such considerations, some authors have found that prognosis and symptomatic benefits are similar between patients with and without AV nodal ablation,15,19,43,44 adding to the controversy surrounding the subject. In a study by Himmel et al., no significant differences were found in functional status, LVEF or left ventricular end-diastolic dimensions between patients with and without AV nodal ablation. This suggests that AV nodal ablation might not be strictly required in all patients with permanent AF and biventricular pacemaker.45
A meta-analysis with similar objectives to ours was previously published,8 but ours also reported cardiovascular mortality in patients with AF and SR, as well as comparing mortality in AF patients who did or did not undergo AV nodal ablation.
In conclusion, the current meta-analysis confirms that CRT should not be ruled out in AF patients as it can have a positive impact on their clinical outcomes, especially when combined with AV nodal ablation.
Study limitationsOur study has several limitations. First, this meta-analysis included mainly observational studies, some with small sample sizes, rather than randomized controlled trials. Thus, we cannot exclude confounding factors as an alternative explanation for our results. Significant differences in baseline characteristics were noted between AF and SR patients in a number of studies. The conclusions of this analysis are limited by the available data. Another possible limitation of our study is the influence of publication bias. However, we included articles with several study designs, as well as abstracts and unpublished studies, so as to reduce the risk of publication bias and improve the sensitivity and power of the meta-analysis.
Significant heterogeneity was present in several analyses and was an important limitation. We applied sensitivity analysis to explore the reasons for heterogeneity.
Further limitations were: differences in total number of patients in each group, with fewer subjects in AF; small numbers of patients who underwent AV nodal ablation; differences in duration of follow-up; inclusion of individuals in paroxysmal, persistent and permanent AF; and the lack of a uniform definition of response to CRT. Finally, the lack of information regarding programming of resynchronization devices and left ventricular lead position may have impaired the homogeneity of the samples.
ConclusionsPatients with HF and AF benefit from CRT, although less than patients in SR. However, the former group is at increased risk of all-cause mortality and shows a higher non-response rate to CRT. AV nodal ablation is associated with a reduction in all-cause and cardiovascular mortality and non-response rate. Therefore, it seems reasonable to consider AV nodal ablation in patients with AF, although the exact extent of the benefits, and the long-term safety of this approach, still remain to be determined. Large randomized trials are needed to confirm our findings.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.