Studies assessing the effects of caffeine (CAF) on the cardiovascular system have demonstrated that CAF can delay cardiac recovery following exercise. This study intended to assess the impact of CAF intake before physical exercise on heart rate variability (HRV) and cardiovascular parameters.
MethodsThis is a prospective, crossover, controlled clinical trial conducted at the University of Pernambuco, Petrolina, PE, Brazil. The experimental protocol was split into three stages with a minimum of 48 hours between them. Exercises intensity was standardized based on the one repetition maximum test (1RM), obtaining the load of each volunteer for the intensity of 75% of 1RM. In the second and third phases, the control protocols were applied and 300 mg caffeine was given 45 minutes before training. HRV indices were determined at the subsequent times: 0 to 5 minutes of rest (before) and during 30 minutes of recovery (Rec) (after exercise), divided into six intervals, each of 5 minutes.
ResultsThe final sample involved 30 volunteers. CAF delayed HRV recovery after resistance exercise. In general, CAF impaired recovery of HRV after resistance exercise. Significant changes were observed in the RMSSD, SDNN, TINN, SD1, low frequency and high frequency indices between the control and CAF group.
ConclusionCAF protocol delayed parasympathetic regulation of heart rhythm following exercise, slowing recovery of HR, blood pressure and HRV indices after exercise.
Estudos avaliando os efeitos da cafeína (CAF) sobre o sistema cardiovascular mostraram que a CAF pode retardar a recuperação dos batimentos cardíacos ao estado de repouso após o exercício. Este estudo teve como objetivo avaliar o impacto da ingestão de cafeína (CAF) antes do exercício físico de força sobre variabilidade da frequência cardíaca (VFC) na recuperação.
MetodologiaTrata-se de um ensaio clínico prospetivo, controlado por cruzamento, realizado na Universidade de Pernambuco, em Petrolina, PE. Está registrado no ClinicalTrials.gov (Number NCT03899675). O procedimento experimental foi dividido em três etapas com um mínimo de 48 horas entre elas. A intensidade do exercício foi padronizada com base no teste de 1 repetição máxima (1RM), obtendo-se a carga de cada voluntário para a intensidade de 75% de 1RM. Na segunda e terceira fase da pesquisa, os protocolos de controle e cafeína (300 mg) foram aplicados. A ingestão de cafeína aconteceu 45 minutos antes do treinamento. Os índices de VFC foram determinados nos seguintes tempos: 0 a 5 minutos de repouso (antes) e durante 30 minutos de recuperação (Rec) (após o exercício), divididos em seis intervalos de 5 minutos cada.
ResultadosA amostra final foi composta por 30 voluntários. No geral, a cafeína prejudicou a recuperação da VFC no período de recuperação após o exercício resistido. Diferenças significativas foram observadas nos índices RMSSD, SDNN, TINN, SD1, LF e HF entre o grupo controle e o grupo CAF.
ConclusãoO protocolo CAF foi capaz de intensificar reduções na atividade parassimpática após o exercício, causando atraso na recuperação da FC, pressão arterial e índices de VFC após o exercício.
The use of supplements by professional athletes, amateurs and recreational practitioners of physical exercise has become common practice, as certain substances promote ergogenic improvement and performance during sporting activities.1 Caffeine (CAF) is categorized as an alkaloid xanthine found in isolation or added to a wide variety of foods and beverages. It has been extensively used in sporting modalities because of its perceived and potential ergogenic effects.2
Its effects are due to the blockage of adenosine receptors (A1 and A2) and increase in the activity of the sympathetic nervous system (SNS) through the release of catecholamines in plasma. At a cardiovascular level, CAF intake promotes tachycardia and elevation of blood pressure (BP)3 during exercise, increases the time to muscle fatigue and attenuates perceived exertion.4 It is, therefore, usual for individuals to take CAF supplements as a strategy for increase training volume and number of repetitions during the series.5–7
Physiological deviations triggered by the combination of CAF and physical exercise may not be favorable when supplementation is used without prior knowledge of cardiovascular parameters and individual characteristics.8 Therefore, depending on the CAF dosage and intensity of physical activity, it can become a contributing factor to physiological difficulties and cardiovascular diseases (CVD), by exacerbating of the activity of the SNS on the physical exercise, specifically in individuals who have CVD.3,9
Considering that the autonomic nervous system (ANS) plays a vital role in controlling blood pressure and heart rate (HR), studying its behavior when stimulated makes it possible to recognize interventions or contributions of certain practices during homeostasis,10 such as CAF intake.11 In this study, we investigate the autonomic modulation of heart rhythm via heart rate variability (HRV), which is a simple and non-invasive method to assess neural control of the heart.12
HRV is one of the most hands-on techniques to analyze the physiological functioning of ANS, both in pathological conditions and with other variables. HRV is a variable that analyzes the peak-R to peak-R (RRi) intervals of consecutive cardiac beats.13 Cardiovascular parameters for example HR, systolic arterial pressure (SAP), and diastolic arterial pressure (DAP) are widely used in cardiovascular health and ANS analysis.14
Consequently, this study attempts to clarify the effects of CAF supplementation prior to exercise on the cardiovascular system. More information concerning the effects of CAF on the ANS triggering heart rhythm disturbances may lead to greater consideration regarding the use of these supplements prior to training; so as to avert injuries and the appearance of CVD in subjects during resistance training. This study sought to assess the impact of CAF intake before strength exercise on HRV and cardiovascular recovery after exercise.
MethodsCONSORT statementOur study conforms to the CONSORT statement, we provided details of the study population and background; subject selection (inclusion/exclusion criteria); methods of randomization and blinding; description of potential sources of bias; efficacy and safety measures; description of quantitative variables and statistical methods with sample size definition.
Study population, inclusion and exclusion criteriaForty-one healthy, college-aged males were chosen for this study. Subjects needed to have had uninterrupted endurance training experience for a minimum of three months and three or more sessions each day. We excluded subjects with the following conditions: neurological, cardiorespiratory, musculoskeletal, endocrine, renal, metabolic and other related disease(s) that would prevent the experimental procedures from being performed, individuals with resting systolic blood pressure >130 mmHg and resting diastolic >90 mmHg, smokers, subjects undergoing pharmacotherapies, sedentary and inadequately active subjects consistent with the International Physical Activity Questionnaire.15
We excluded subjects who stated they used anabolic steroids. Those who presented series of RR intervals with under 95% of sinus beats and those who were unable to complete all stages of the protocols (Figure 1).
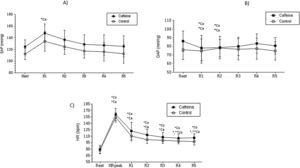
Mean values and respective standard deviation of systolic arterial pressure (A), diastolic arterial pressure (B) and heart rate (C) obtained in the control and caffeine protocols at rest and during recovery (Rec).
Ca: Caffeine; Co: Control; HR Peak: maximum heart rate value during exercise. R1: 5th minute of rec; R2: 10th minute of rec; R3: 15th minute of rec; R4: 20th minute of rec and; R5: 30th minute of rec.
*Co: p<0.05 vs. Rest in Control protocol; *Ca: p<0.05 vs. Rest in Caffeine protocol; **Ca: p<0.05 vs. R1 in Caffeine protocol (n=30 subjects).
Informed consent was signed by all subjects before the tests. Experimental procedures were approved by the Research Ethics Committee of University of Pernambuco, Recife, PE, Brazil (Number 3.056.402) and were in agreement with the 466/2012 resolution of the National Health Council of 12 December 2012.
Study design and settingThis is a prospective, crossover and controlled trial performed at the University of Pernambuco, Recife, PE, Brazil. The study was registered with ClinicalTrials.gov (NCT03899675).
Initial assessment and experimental protocolsEfficacy and safety measuresThe protocols were completed at two distinct times between 16:30 and 21:00 in a noiseless room with controlled temperature between 22°C and 25°C and humidity between 60% and 70%. Subjects were instructed to avoid drinking alcohol or performing exhaustive exercise 24 hours prior to the evaluation and to abstain from food or CAF drinks eight hours prior. Also, subjects were advised to eat only a light meal three hours prior to the procedures and to wear comfortable clothing suitable for the necessary physical exertion.
BiasSo as to define potential sources of bias, the descriptive profile of the subjects was defined to characterize the sample, reduce the volatility of the variables, improving reproducibility and physiological interpretation.
Before the start of the experimental procedures, subjects were recognized according to age, mass (kg), height (m), systolic (mmHg) and diastolic arterial pressure (mmHg), waist (cm), abdominal (cm) and hip (cm) circumferences, waist-to-hip ratio and body mass index (BMI).
Experimental protocolsThe experimental procedure was split into three stages with a minimum interval of 48 hours between them, so as to allow the subjects adequate recovery time. On the first day, the physical exercise intensity test was performed based on the 1RM test, which was completed before the other stages, as it was necessary to prescribe the exercise intensity in the others. In the second and third phase of the research, the control and CAF protocols were performed. The subsequent phases were the control and CAF protocols and the order in which they were implemented was established through a randomization process involving the tossing of a coin. The subjects were blinded throughout their protocol and were uninformed about the order of the protocols
Before the start of the first stage, body weight measurements on a digital scale (Welmy, W200/5, São Paulo, Brazil) and height using a stadiometer (ES 2020 - Sanny, São Paulo, Brazil) were recorded.
Training intensity was defined based on the maximal 1RM following the recommendations of the American College of Sports Medicines’ Guidelines for Exercise Testing and Prescription.16 Subjects were coached to test the load in the four proposed physical exercises with 1RM performed in the subsequent exercises:
- 1.
Leg press 45°,
- 2.
Extending chair,
- 3.
Abductor chair and
- 4.
Squatting.
On subsequent days of data collection, the exercises were performed based on the load of 75% 1RM for the aforesaid exercises, based on the load from the test 75% 1RM previously performed, a total of four sets were completed with a maximum of 10 repetitions for each exercise.
CAF and control protocolPrior to starting protocol, the HR monitor (Polar RS800CX, Finland) was positioned on the subjects to register HR beat-to-beat, followed by administration of a 300 mg CAF capsule or a placebo;according to the selected protocol. We set the dose of CAF at 300 mg as this is considered safe. The 2015-2020 Dietary Guidelines for Americans allow up to a maximum of 400 mg/day,17 whilst the United States Food and Drug Administration specifies that 300 mg is within the maximum allowed per day.18
Resting phase: Initially, the subjects were seated, at rest, for 5 minutes, whilst recording HR, SAP and DAP values measured in the last minute of the resting phase. Similarly, in this phase, HRV indices were recorded for 5 continuous minutes (rest interval).
Exercise: After these measurements the subjects performed strength exercise at 75% of 1 RM.
Recovery phase: At the end of the activity, the subjects were again located seated at rest, and were observed for 30 minutes. HR, SAP and DAP values were recorded within five, 10, 15, 20, and 30 minutes of recovery. HRV indices were recorded for 30 minutes of recovery with six intervals of 5 minutes each between them: Rec1 (0 to five minutes), Rec2 (five to 10 minutes), Rec3 (10 to 15 minutes), Rec4 (15 to 20 minutes), Rec5 (20 to 25 minutes) and Rec6 (25 to 30 minutes).” To avoid measurement errors in the assessed parameters, a single rater performed all measurements throughout the whole experiment.
The control phase was completed on the second day of collection, in which subjects did not ingest CAF. The datasets were collected in the standardized way, respecting the times established for the analysis of cardiac activity. Nevertheless, before the tests the subjects were questioned about the use of CAF. At the end of the first day of testing (control), subjects received a capsule containing 300 mg of CAF and in accordance with the earlier guidelines, were instructed to ingest the capsule 45 minutes before the next day of procedure, giving the subjects adequate time for digestion and absorption of the CAF.
Blood pressureSAP and DAP were checked indirectly using a stethoscope (Littman Classic II, Saint Paul, USA) and aneroid sphygmomanometer (Welch Allyn Tycos, New York, USA) placed on the subjects’ left arm.19
Analysis of heart rate variabilityHRV analysis was performed throughout the entire experimental protocol using an HR monitor (Polar RS800CX, Finland), equipment validated formerly to record HR.
The recordings contained the required 256 consecutive stable RR intervals, which underwent digital filtering accompanied by manual filtering to eliminate artifacts. Only series greater than 95% of sinus HR were included in the study. Analysis of HRV linear methods in the time domain and the frequency were applied. In the time domain indexes square root of the average of the square of the differences between normal RR intervals adjacent (RMSSD) and standard deviation of the average of all normal RR intervals (SDNN) were necessary. The Poincaré plot indexes: standard deviation of the instantaneous rate variability the rhythm (SD1) and standard deviation of long-term continuous RR interval variability (SD2)and Geometric Index: Triangular index (RRTri)and Triangular interpolation of NN interval histogram (TINN) were also calculated.10
For HRV frequency domain analysis, the spectrum components low frequency and high frequency, in ms2 and in normalized units, extracted using the Fast Fourier Transform (Blackman & Tukey, FFT) were used. The frequency bands required for each component were: low frequency (LF: 0.04 to 0.15 Hz) and high frequency (HF: 0.15 to 0.40 Hz). We decided not to evaluate the LF/HF ratio as it has been demonstrated to be theoretically flawed and empirically unsupported to represent the sympathovagal balance.20
HRV analysis software (Kubios HRV®, Biosignal Analysis and Medical Image Group, Department of Physics, University of Kuopio, Finland)21 was used to scrutinize the linear indices in the time and frequency domains and to perform the Poincaré plots.
Data analysisTo describe sample size, a sample calculation was completed based on the study by Gonzaga et al.3 considering the RMSSD index. The assumed significant difference magnitude was 12 ms, in view of a standard deviation of 16.2 ms, with alpha risk of 5% and beta of 80%. A total of 28 subjects were required for the study.
Regarding the data analysis, we enforced the descriptive statistics on the characterization of the sample and the results were reported as mean values, standard deviation, minimum and maximum. Comparisons of the HRV indices and cardiovascular parameters between CAF and Control protocols; moments were achieved via two-way repeated measures analysis of variance. For analysis of the moments (rest versus recovery), we implemented the Bonferroni post-test for parametric distribution or post-test Dunn for non-parametric distribution. Statistical significance was set at p<0.05 (or <5%) throughout.
Computations were completed using Minitab version 13.20 (Minitab, PA, USA), GraphPad Instat version 3.01, 1998 (GraphPad Software, Inc., San Diego, California, USA) and IBM SPSS Statistics version 22.0 (SPSS Inc., Chicago, IL, USA).
ResultsAltogether, 30 subjects contributed to the research study. The results of their anthropometric characteristics and experience with physical strength training are illustrated in Table 1.
Mean values, followed by their respective standard deviations, minimum and maximum values of the anthropometric variables and the experience with exercise.
| Variables | Mean±SD | Minimum-maximum |
|---|---|---|
| Age (years) | 23.33±3.15 | [19-27] |
| Height (m) | 1.63±4.51 | [1.59-1.96] |
| Weight (kg) | 71.14±12.31 | [55-97] |
| BMI (kg/m2) | 23.00±2.75 | [17.96-27.15] |
| Experience Time in Strength Exercise (months) | 10.80±9.44 | [3-36] |
Legend: BMI: body mass index; kg: kilogram; m: meter; SD: standard deviation.
We did not find changes between the groups by examining the resting HR, however, in the CAF group, HR was superior in all recovery period intervals. After the 5th minute of recovery, systolic blood pressure in the CAF cohort was found to be significantly higher when compared to the control group (CAF vs Control: SAP 148.00±15.68 vs 134.00±16.82 p=<0.05) (Figure 1).
Differences between heart rate variability indexOn the whole, CAF impaired HRV recovery after resistance exercise. Significant differences were observed in the RMSSD (REC 5 vs. REC1** and REC 5 vs. REC 2*** p<0.001; REC 6 vs. REC1** and REC 6 vs. REC2*** p<0.001), SDNN (REC 5 vs. REC1** and REC 5 vs. REC 2*** p<0.001; REC 6 vs. REC1** and REC 6 vs. REC2*** p<0.001) (Figure 2), TINN (REC 4 vs. REST* p<0.001); REC 6 vs. REC1** p<0.0001 and REC 6 vs. REC2*** p<0.001) (Figure 3), SD1 (REC5 vs. REC1** p>0.0001 and REC 5 vs. REC2*** p>0.0001), SD2 (REC 5 vs. REST* p>0.0001; REC 5 vs. REC 1** p>0.0001 and REC 5 vs. REC2*** p>0.0001) and REC 6 vs. REC 1** p<0.0001 and REC 6 vs. REC 2*** p<0.0001) (Figure 4), LF (REC 5 vs., REC1** p=0.0005 and REC 5 vs., REC2*** p=0.0004) and REC 6 vs. REC1** p = 0.004 and REC 6 vs. REC 2 p = 0.0004), HF (REC 6 vs. REST* p=0.007) indices between the control group and the CAF group. The results of this study indicate that CAF in combination with exercise strength can impair vagal recovery for a period of 30 minutes (Figure 5).
Mean values and respective standard deviations of time domain HRV indices obtained in the control and caffeine protocols at rest and during recovery (Rec).
Ca: Caffeine; Co: Control; RMSSD: root-mean square of differences between adjacent normal RR intervals in a time interval; SDNN: standard deviation of all normal RR intervals; ms: milliseconds;
*Co: p<0.05 vs. Rest in Control protocol; *Ca: p<0.05 vs. Rest in Caffeine protocol; **Co: p<0.05 vs. Rec 1 in Control protocol; **Ca: p<0.05 vs. R1 in Caffeine protocol; ***Ca: p<0.05 vs. Rec 2 in Caffeine protocol (n=30 subjects).
Mean values and respective standard deviations of geometric HRV indices obtained in the control and caffeine protocols at rest and during recovery (Rec). Ca: Caffeine; Co: Control; ms: milliseconds; RRTri: triangular index; TINN: triangular interpolation of RR interval histogram; *Co: p<0.05 vs Rest in Control protocol; *Ca: p<0.05 vs. Rest in Caffeine protocol; **Ca: p<0.05 vs. Rec 1 in Caffeine protocol; ***Ca: p<0.05 vs. Rec 2 in Caffeine protocol (n=30 subjects).
Mean values and respective standard deviations of the Poincaré HRV indices obtained in the control and caffeine protocols at rest and during recovery (Rec).
Ca: Caffeine; Co: Control; ms: milliseconds; SD1: standard deviation of the instantaneous variability of the beat-to-beat heart rate; SD2: standard deviation of long-term continuous RR interval variability. *Co: p<0.05 vs. Rest in Control protocol; *Ca: p<0.05 vs. Rest in Caffeine protocol; **Co: p<0.05 vs. Rec1 in Control protocol; **Ca: p<0.05 vs. Rec1 in Caffeine protocol; ***Ca: p<0.05 vs. Rec2 in Caffeine protocol (n=30 subjects).
Mean values and respective standard deviations of frequency domain HRV indices obtained in the control and caffeine protocols at rest and during recovery (Rec). LF: low frequency; HF: high frequency; ms: milliseconds; Ca: Caffeine; Co: Control; *Co: p<0.05 vs Rest in Control protocol; *Ca: p<0.05 vs Rest in Caffeine protocol; **Co: p<0.05 vs Rec1 in Control protocol; **Ca: p<0.05 vs Rec1 in Caffeine protocol; ***Ca: p<0.05 vs Rec2 in Caffeine protocol (n=30 subjects).
HRV reduction is perceptible in the decrease of SDNN, as observed in the CAF group even 30 minutes after recovery (REC 6). CAF similarly delayed the recovery of the frequency domain indices LF and HF, where we found significant differences between the groups. Slow recovery of HF establishes that CAF delivers a reduction in parasympathetic conduction for a period of 30 minutes after exercise cessation (Rest vs. REC 6).
The RRtri, TINN and SD2 geometric indices were affected by CAF intake prior to exercise, meaning that its use enables a lower ANS adaptation to the stimulus of physical exercise, interpreted by an overactivity of the sympathetic response to exercise. From this viewpoint, in the CAF group, TINN showed delayed recovery until the last moment (REC 4, 5 and 6). The recovery of SD2 in the CAF group was late; this parameter was significantly reduced during the final intervals of the recovery period (REC 5 and REC 6).
DiscussionOur results confirm that ingestion of CAF (300 mg) before strength exercise: (a) impaired the recovery of vagal HR control during recovery after exercise; (b) delayed the recovery of SAP after physical exertion; (c) delayed HR recovery to baseline resting levels and no significant deviations were recognized for DAP.
Soon after strength exercise cessation, we identified that the HF (REC 6) of the CAF group experienced delayed recovery. This emphasizes that there was a greater vagal withdrawal through exercise and preservation of the sympathetic drive in the recovery period after exercise. The HF component is reduced when the autonomic recovery of HR is affected.22 In this way, CAF substantially affected the autonomic return of parasympathetic activity.
At the time of recovery after exercise, CAF delayed vagal recovery after exercise termination. We identified these results by means of index analysis and their respective recovery moments, SDNN (REC 5, REC6), TINN (REC 6), SD2 (REC 5 and REC 6), LF (REC 5 and REC 6) and HF (REC 6). Thus, it was found that CAF affects the recovery until the protocol is completed (30 minutes). Further studies have revealed that these effects are also identified in aerobic exercise.3,23
With this in mind, the potential effects of CAF have been studied for several decades in various sports and the perceived effects of CAF is the key reason for its use in recreational sports.24 Yet, there is currently a new outlook on the intake of CAF prior to physical training. The effects of CAF on ANS have been tested in several sports modalities and practices.25,26 The results described by other studies applied mostly to aerobic exercise (cycling, treadmill test). Studies concentrating on HRV and CAF in advance of strength exercise are rare in the research literature.
The influence of CAF on HRV in strength exercise has already been studied, but in this case, CAF was administered in combination with taurine in an energy beverage. It is expected that taurine has antagonistic effects on CAF and owing to this mechanism, the results are inconsistent with the research literature. Although the study did not report any alterations in HRV, there was an increase in HR before resistive physical exercise.27 In this study, we did not detect significant differences in resting HR between the two groups. However, SAP was significantly higher in the CAF group.
During physical activity blood pressure increases because of a need for greater oxygenation to permeate the tissues; cessation of physical exercise enables SAP to return to resting levels. Smirmaul et al.,28 reported that CAF supplementation delays SAP values to the resting baseline. This effect was confirmed in this study; the SAP of the CAF group was greater at the moments inthe recovery period following physical exercise.
The acute response to blood pressure control is mediated by baroceptors. CAF seems to affect this mechanism by encouraging the permanence of high levels (>120 mmHg/80 mmHg) after cessation of physical exercise. Hypertensive consequences were observed in this study, after 5 minutes of recovery, in the CAF group.
The analysis of CAF's effect on autonomic parameters is divided into three moments: before, during and after physical exercise. At the time before physical activity, some datasets have shown contentious results for the breakdown of autonomic outcomes. Kliszczewicz et al.11 found an increase in sympathetic activity during the rest period with 100 mg of CAF. Yet, even with 300 mg administered in the study by Gonzaga et al.3 (2017), no significant changes were observed between the two groups at rest.
Throughout exercise, there is a greater intensity of vagal withdrawal when CAF is ingested. This outcome is clear, shown by an increase in HRpeak, which is explained in the majority of studies that assess HR during physical exercise. Also, increased SAP during exercise is highlighted with CAF intake.23 This is due to the vasoconstriction caused by CAF, partially by the increased release of catecholamines into the bloodstream.
In this scenario, when recovering from exercise, CAF appears to affect the bradycardic effect in response to the increase in blood pressure, considering that in the moments of recovery, both HR and SBP remain elevated for at least 5 minutes after the end of the exercise. These effects were not seen in the control group (Figure 1).
We suggest that these conditions are caused mainly by an increase in vagal withdrawal and the amount of catecholamines released into the bloodstream during exercise. In this scenario, other studies report that there is an increased release of catecholamines with CAF intake29; and concerning this, the greater the amount of catecholamines the slower the recovery of cardiovascular activity after exercise.30,31
Thus, the study conducted by Gonzaga et al.3 assessing 32 young adult males had similar outcomes to our own. The ingestion of 300 mg of CAF in combination with submaximal aerobic exercise (65% to 75% of HRmax) encouraged a delay in the parasympathetic reactivation for a period of up to one hour; these results were evaluated by the RMSSD, SD1, SD2 indices.
This effect was similarly clarified in the experiment by Bunsawat et al.,23 in which 400 mg intake of CAF was shown to delay vagal reactivation after exercise for the maximum time (30 minutes), when subjects were assessed during the recovery period. Additionally, systolic and diastolic blood pressures were higher than in the control group.
One of the major concerns that orients the use of CAF before exercise is its unselective use. Since CAF has thermogenic effects, it is common for overweight or obese people to resort to CAF supplementation as a way to heighten weight loss.
The effects of CAF on the autonomic and cardiovascular parameters of overweight or obese individuals are unknown, since the effects found in the already completed studies were tested in physically active individuals or athletes. Bearing in mind that the neural adaptation of the their cardiovascular system to different situations is better, it is conjectured that the effects of CAF on overweight or obese subjects are even more deleterious.
ConclusionCAF delayed HR and HRV recovery following strength exercise. In view of these findings, we emphasize that prior to CAF use, a clinical assessment of cardiovascular and autonomic parameters by a medical physician should be performed to provide individual recommendations.
FundingCicero Jonas R. Benjamim receives financial support from Pernambuco State Foundation for Science and Technology - FACEPE (Process number: BIC: 0404-4.09/19 and BIC: 1844-4.05/20). Vitor E. Valenti receives financial support from Foundation of Support to Research from Sao Paulo State - FAPESP (Process number: 2016/02994-1) and the National Council for Scientific and Technological Development, an entity linked to the Ministry of Science, Technology, Innovations and Communications from Brazil (Process number: 302197/2018-4).
Conflicts of interestThe authors have no conflicts of interest to declare.