The optimal length of stay for patients with uncomplicated ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PPCI) is still undetermined. The Zwolle risk score (ZRS) is a simple tool designed to identify patients who can be safely discharged within 72 hours. The purpose of this study was to assess the applicability and performance of the ZRS in our population.
MethodsWe studied 276 consecutive patients (mean age 62±14 years, 75% male, 20% Killip class >1) admitted over a two-year period for STEMI and treated with PPCI. ZRS, length of stay, 30-day mortality and readmission were obtained for all patients. Low risk was defined as ZRS ≤3.
ResultsThe median ZRS was 3 (interquartile range [IQR] 1–4), with 171 patients (62%) being classified as low risk. Thirty-day mortality was 4.7% (13 patients). Compared to other patients, low-risk patients had shorter length of stay (median 5.0 [IQR 4–7] vs. 7.0 [5–13] days, p<0.001), and lower 30-day mortality (0 vs. 12.4%, p<0.001), yielding a negative predictive value of 100% (95% CI 97.0–100%) for the proposed cutoff. The ZRS showed excellent discriminative power (C-statistic: 0.937, 95% CI 0.906–0.968, p<0.001), and good calibration against the original cohort.
ConclusionsThe ZRS appears to perform well in identifying low-risk STEMI patients who could be safely discharged within 72 hours of admission. Using the ZRS in our population could result in a more rational use of in-patient resources.
A duração ótima de internamento após enfarte agudo do miocárdio com supradesnivelamento do segmento ST (EAMCST) não complicado, submetido a intervenção coronária percutânea primária (ICPP), permanece por determinar. O Score de Zwolle (SZ) é um instrumento simples, desenhado para identificar os doentes candidatos a alta precoce (<72h) segura. Este estudo pretendeu avaliar a aplicabilidade do SZ na nossa população.
MétodosAnalisámos 276 doentes consecutivos (idade média 62±14 anos, 75% homens, 20% em classe de Killip>1) com EAMCST submetidos a ICPP durante um período de dois anos. Foram obtidos os SZ, duração de internamento, mortalidade e readmissão aos 30 dias. Foi definido baixo risco como SZ ≤3.
ResultadosA mediana do SZ foi de 3 [distância interquartil (IQR) 1-4] e 171 doentes (62%) foram classificados como de baixo risco. A mortalidade aos 30 dias foi de 4,7% (13 doentes). Em comparação com os restantes, os doentes de baixo risco tiveram menor duração de internamento [mediana 5,0 (IQR 4-7) versus 7,0 (IQR 5-13) dias, p<0,001] e menor mortalidade (0 versus 12,4%, p<0,001), resultando num valor preditivo negativo de 100% (IC95% 97,0-100%) para o cut-off proposto. O ZS mostrou excelente poder descriminativo (estatística-C: 0,937, IC95% 0,906-0,968, p<0,001) e boa calibração quando comparado com o coorte original.
ConclusõesO SZ parece capaz de identificar com precisão os doentes com EAMCST de baixo risco que podem ter alta segura 72h após a admissão. O uso do SZ na nossa população poderá resultar numa utilização mais racional dos recursos hospitalares.
ST-segment elevation myocardial infarction (STEMI) is one the most frequent presentations of acute coronary syndromes. Despite significant reductions in morbidity and mortality due to optimization of reperfusion strategies, some issues are still poorly defined in the current guidelines.1,2 One of the least studied aspects is the optimal length of stay (LOS) for patients with uncomplicated STEMI. Current practice adopts 5–7 days for monitoring of complications, but this is largely empirical.3,4 A need to better define the appropriate LOS has been recognized by European and American cardiovascular societies, which suggest the use of prognostic tools to accurately identify low-risk patients who can be safely discharged within 72 hours.1,2,5,6 The Zwolle risk score (ZRS) is a simple six-item score (age ≥60, time to reperfusion >4 hours, anterior infarct, TIMI flow post angioplasty, three-vessel disease, Killip class; Appendix 1) that showed promising results in a pivotal study.7 Using the ZRS could potentially improve patient throughput and reduce healthcare costs,5,7 but any failure to identify patients who will suffer from early complications could have dire consequences and legal implications. The adoption of a targeted early discharge policy should therefore always be preceded by validation in the specific population in which it will be used. We sought to validate the ZRS in our population of STEMI patients and to identify variables associated with increased LOS.
MethodsWe conducted an observational retrospective study of all patients with STEMI referred to our center for primary percutaneous coronary intervention (PPCI) between January 2011 and December 2012 (n=278). Of these, two died during the index procedure and were excluded from the analysis, yielding a population of 276 patients admitted to the cardiac intensive care unit. The ZRS was calculated for each patient.7 In this score, each variable is given points for a minimum score of 0 and a maximum of 16 (Appendix 1). Low risk was defined as ZRS ≤3, as in the original study.7 In the subset of low-risk patients, clinical data were additionally scanned for variables that could influence LOS. LOS was defined as the time (in days) from first balloon inflation to time of hospital discharge. The primary endpoint was 30-day mortality. The secondary endpoint was death or readmission within 30 days of discharge. Mortality and readmissions up to 30 days after discharge were obtained for all patients by reviewing medical charts, supplemented by telephone interview whenever necessary. Informed consent for inclusion in a prospective registry was obtained from all patients.
The statistical analysis was performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA) and MedCalc 6.0 (MedCalc Software, Ostend, Belgium). Differences between groups were tested using the t test and one-way analysis of variance and the K test for medians for continuous variables with a normal and non-normal distribution, respectively. Categorical variables were compared using Fisher's exact test. The log-rank test was used for survival analysis. The clinical usefulness of any predictive score or model relies on its goodness-of-fit and discriminative ability.8 The goodness-of-fit of the ZRS, reflecting how close the predicted 30-day mortality is to that observed, was assessed by a calibration plot and by the calibration-in-the-large statistic.8 The discriminative ability of the ZRS in our population (i.e., the extent to which the score is able to separate survivors from non-survivors) was expressed by the C-statistic, which, for binary outcomes, is equal to the area under the receiver operating characteristic (ROC) curve. Two-tailed p values <0.05 were considered statistically significant.
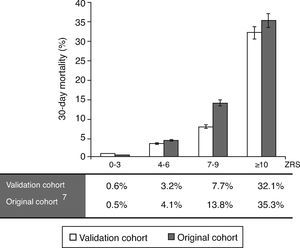
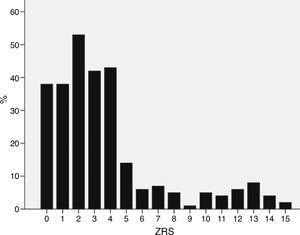
ResultsPatient characteristicsPatient characteristics are described in Table 1. The median ZRS was 3 (interquartile range [IQR] 1–4), with 171 patients (62%) being classified as low-risk (Figure 1). Compared to other patients, low-risk patients were younger and had a lower prevalence of hypertension and diabetes and a higher proportion of smokers. The frequency of each component of the ZRS is depicted in Table 2.
Population characteristics.
| ZRS ≤3 (n=171) | ZRS >3 (n=105) | p | ||
|---|---|---|---|---|
| Age, years (mean ± SD) | 62.0±33 | 57.9±13.0 | 68.8±11.0 | <0.001 |
| Male (%) | 208 (75.4) | 133 (77.3) | 75 (72.1) | 0.387 |
| Hypertension (%) | 151 (54.7) | 81 (47.1) | 70 (67.3) | 0.001 |
| Dyslipidemia (%) | 121 (43.8) | 75 (44.2) | 46 (43.6) | 1.0 |
| Diabetes (%) | 54 (19.6) | 25 (14.5) | 29 (27.9) | 0.008 |
| Smoking (current or past) (%) | 153 (55.4) | 116 (67.4) | 37 (35.6) | <0.001 |
| Previous MI (%) | 39 (14.1) | 19 (11.0) | 20 (19.2) | 0.074 |
| Previous PCI (%) | 36 (13.0) | 18 (10.5) | 18 (17.3) | 0.139 |
| Previous CABG (%) | 8 (2.9) | 3 (1.7) | 5 (4.8) | 0.158 |
| GRACE scorea (median, IQR) | 147 (130–170) | 144 (129–63) | 155 (136–179) | 0.139 |
| Ischemia time (median, IQR) | 4.7 (2.9–7.9) | 4.0 (2.5–6.1) | 6.3 (3.9–9.6) | <0.001 |
| TIMI flow pre-angioplasty (median, IQR) | 0 (0–2) | 0 (0–3) | 0 (0–2) | 0.704 |
| Stent (%) | 240 (87.0) | 153 (89.5) | 87 (82.9) | 0.141 |
| Mean no. of stents per patient | 1.07 | 1.06 | 1.09 | 0.364 |
| Procedural success (%) | 98 | 98 | 95 | 0.129 |
| Peak troponin I, μg/l (median, IQR) | 43 (10–102) | 41 (11–99) | 51 (7–111) | 0.442 |
| LVEF ± SDb (%) before discharge (%) | 52.0 ± 9.8 | 54.0 ± 8.2 | 48.7 11.3 | 0.001 |
| LOS in days (median, IQR) | 5.0 (4–9) | 5.0 (4–7) | 7.0 (5–13) | <0.001 |
CABG: coronary artery bypass grafting; IQR: interquartile range; LOS: length of stay; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; ZRS: Zwolle risk score.
Distribution of individual variables of the Zwolle risk score.
| n (%) | ZRS ≤3 (%) | ZRS >3 (%) | p | |
|---|---|---|---|---|
| Killip class | ||||
| I | 220 (80) | 99 | 52 | <0.001 |
| II | 31 (11) | 1.0 | 0 | |
| III | 7 (2.5) | 0 | 21 | |
| IV | 18 (6.5) | 0 | 27 | |
| TIMI flow post-reperfusion | ||||
| 3 | 255 (92) | 98 | 84 | <0.001 |
| 2 | 7 (2.5) | 0.6 | 7 | |
| 0–1 | 14 (5.1) | 0.4 | 9 | |
| Age ≥60 years | 150 (54) | 37 | 81 | <0.001 |
| Three-vessel disease | 48 (17) | 11 | 29 | <0.001 |
| Anterior MI | 134 (49) | 40 | 65 | <0.001 |
| Ischemia time >4 hours | 167 (61) | 51 | 77 | <0.001 |
MI: myocardial infarction; ZRS: Zwolle risk score.
Thirty-day mortality occurred in 13 patients (4.7%). ROC curve analysis yielded a C-statistic of 0.937 (95% CI 0.906–0.968, p<0.001), demonstrating excellent discriminative power (Figure 2).
Calibration-in-the-large was performed using the observed mortality rate of the original study7 compared against this validation cohort, divided into four subgroups (Figure 3), yielding a value of 10.1%, due to a higher mortality rate in the original cohort. Overall, the observed mortality was similar to that predicted by the ZRS, although the latter tended to overestimate the likelihood of fatal events in patients with higher scores.
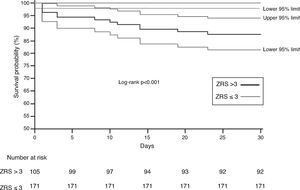
When stratified using a cut-off ZRS of 3, a significant difference was found in 30-day survival between patients classified as ZRS ≤3 and ZRS >3 (100 vs. 87.6%, log-rank p<0.0001; Figure 4), yielding a negative predictive value of 100% (95% CI 97–100%) for 30-day mortality.
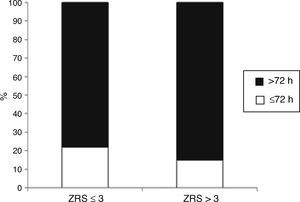
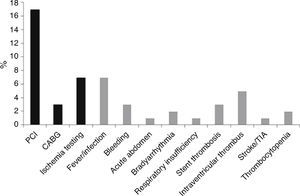
Length of stayMedian LOS was 5 (IQR 4–9) days. Median LOS in the low-risk group was significantly lower (5.0 [IQR 4–7] vs. 7.0 [IQR 5–13] days, p<0.001), with 22% of patients being discharged within 72 hours in the low-risk group vs. 14% in the non-low-risk group (Figure 5). In the 171 low-risk patients, 38 were discharged in ≤72 hours after the index event. Among the other 133 individuals, 52 had some contraindication for an earlier discharge (e.g. left ventricular thrombus, atrial fibrillation, or bleeding complication). Additional staged revascularization (either percutaneous or surgical) was the most frequent cause for a lengthened stay in this low-risk population. Seven patients also underwent additional ischemia testing. In this low-risk cohort, 81 patients had no discernible medical reason for an increased LOS. These patients represented 221 extra days of stay beyond 72 hours (Figure 6).
No deaths occurred in the 30 days following discharge. Four (1.4%) patients were readmitted in this period (Table 3), three in the ZRS ≤3 group and one in the ZRS >3 group (p=1.0).
DiscussionIn the absence of formal recommendations on the ideal LOS after uncomplicated STEMI, decisions regarding the time of discharge are usually made on an individual basis, considering the patient's previous comorbidities and immediate clinical course following MI. Our study shows that, in a population of real-world consecutive STEMI patients treated with PPCI, a strategy of early discharge (≤72 hours) guided by the ZRS is feasible and safe in selected low-risk patients. None of these low-risk patients died, and their readmission rate was not significantly higher than in non-low-risk individuals. Importantly, none of the complications that prompted readmissions occurred in the more usual five-day period of hospital stay. The ZRS showed excellent discriminative ability and good fit in our population, suggesting that it could be used to guide early discharge in appropriately selected low-risk patients.
Over recent decades, prognosis after STEMI has improved, owing mainly to the development of modern reperfusion modalities and antithrombotic drugs. Notably, LOS has also seen a progressive reduction, without an impact on mortality.9–11 This observation is particularly true for primary reperfusion in MI. Recent STEMI guidelines have stated, although with a low level of recommendation (IIB, level of evidence B), that risk score assessment tools such as the Prognosis Assessment in Myocardial Infarction (PAMI) score or the ZRS can be used to assess the risk for early discharge (within approximately 72 hours) and that this strategy is reasonable in selected low-risk patients, if early rehabilitation and appropriate follow-up are arranged. Historically, LOS after acute MI has evolved from an era of prolonged bed rest and rehabilitation of over six weeks to the present LOS of less than one week.10 The typical LOS in the USA is five days in uncomplicated MI.12 A number of studies have shown that a shorter LOS in acute MI, especially in the PPCI era, has not increased early cardiovascular mortality, recurrent ischemia or unplanned readmission due to heart failure.11–14 This study strengthens that notion, suggesting that in a large-volume PPCI center, there is a significant proportion (62.6%) of patients admitted for percutaneous revascularization deemed to have a low mortality risk.
The rationale supporting an early discharge relates to the majority of life-threatening complications occurring in the first 72 hours.6 Secondary malignant ventricular arrhythmias, mechanical complications and pump failure associated with extensive areas of myocardial necrosis usually occur in this time window, providing support for such a strategy. Topol et al. were among the first to demonstrate the safety of an early discharge policy, in 179 patients with uncomplicated MI (no angina, heart failure, or arrhythmia 72 hours after admission).15 In another study, Newby et al. used a mixed-exponential survival model (an acute curve for most adverse events and a chronic curve describing a lower background rate) to show that up to 97% of complications (death, stroke, shock, congestive heart failure, reinfarction or ischemia) occur within 72 hours of admission for acute myocardial infarction (MI).6 Moreover, the authors concluded that hospitalization of patients with uncomplicated MI beyond three days after thrombolysis was economically unattractive by conventional standards.
The second question we aimed to answer pertains to the reasons behind increased LOS in this low-risk population. Fifty-two patients (30.4% of the low-risk cohort) were not discharged early, based on either a contraindication (18.7%) or a scheduled intervention (i.e. additional revascularization, 11.7%). These results are in agreement with the 16.1% of patients with contraindication to early discharge found in the study by De Luca et al.7 The decision to proceed with revascularization during the index admission (whether surgical or percutaneous) was tailored to each individual's case, taking into account the severity of angiographic lesions, myocardial area at risk and perceived risk-benefit relationship, as assessed by the medical team. Recently, the PRAMI trial suggested that non-culprit vessel revascularization during PPCI could be beneficial. If confirmed in larger trials and widely adopted, this strategy could indirectly help to reduce overall LOS.16 Our data also support the notion that LOS is driven to some extent by non-clinical factors and that there is room for improvement. Other logistical and institutional factors, such as weekends and days with less medical staff (e.g. due to other activities such as teaching) have previously been identified as reasons for a prolonged LOS.17
Some authors have raised concerns about early discharge after STEMI.2,18,19 The potential disadvantages of this strategy include less time for patient education (e.g. smoking cessation), drug titration and cardiac rehabilitation.17 Notwithstanding these concerns, a study by Kotowycz et al. showed that drug adherence and smoking cessation rates among acute MI patients who were discharged early did not differ between those allocated to close post-discharge follow-up compared to those undergoing normal follow-up.3 Regardless of policy, the use of risk assessment scores should never replace careful clinical evaluation, for which echocardiographic and angiographic data provide essential information.
Several limitations of this study should be acknowledged. Firstly, this was a single-center, retrospective analysis, subject to inherent selection biases and sample size-related statistical limitations. Secondly, variables known to influence the prognosis of STEMI patients, such as time from symptom onset to first medical contact, admission brain natriuretic peptide or ST-segment resolution, were unavailable in a significant proportion of patients and were thus not included. Thirdly, this study does not address cost-effectiveness issues. Further studies are warranted to assess the economic impact of adopting a ZRS-guided early discharge policy.
ConclusionsIn our population, the ZRS showed excellent discriminative capability to identify low-risk STEMI patients who could be safely discharged within 72 hours of PPCI. Further strategies are needed to optimize LOS in low-risk patients without a contraindication for early discharge.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.














![Receiver operating characteristic curve for Zwolle risk score and 30-day mortality (C-statistic=0.937 [95% confidence interval 0.906–0.968, p<0.001]).](https://static.elsevier.es/multimedia/21742049/0000003400000009/v1_201509130054/S2174204915001853/v1_201509130054/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)