To analyze the percentage of collagen fibers and mast cell density in the left ventricular myocardium of autopsied patients with and without hypertensive heart disease.
MethodsThirty fragments of left ventricular myocardium were obtained from individuals autopsied at the Clinical Hospital of the Federal University of Triângulo Mineiro (UFTM) in the period from 1987 to 2017. Individuals were divided into two groups: those with hypertensive heart disease (HD) and those with no heart disease (ND). Subjects were also assessed according to age, gender and race (white and non-white). Collagen fibers were quantified by computed morphometry and mast cell density was assessed by immunohistochemical methods.
ResultsThere were significantly more collagen fibers in the left ventricle in the HD group than in the ND group (p<0.001). Mast cell density was significantly higher in the left ventricle of individuals with HD immunolabeled with anti-chymase and anti-tryptase antibodies (p=0.02) and also of those immunolabeled only with anti-tryptase antibodies (p=0.03). Analyzing the HD group, there was a significant positive correlation between the percentage of collagen fibers in the left ventricle and mast cell density immunolabeled by anti-chymase and anti-tryptase antibodies (p=0.04) and also mast cell density immunolabeled only with anti-tryptase antibodies (p=0.02).
ConclusionsMast cells are involved in the development of hypertensive heart disease, contributing to the remodeling of collagen fibers in this disease.
Analisar a porcentagem de fibras colágenas e a densidade mastocitária no miocárdio ventricular esquerdo de pacientes autopsiados com e sem cardiopatia hipertensiva.
MétodosForam obtidos 30 fragmentos do miocárdio ventricular esquerdo de indivíduos autopsiados no Hospital Clínico da Universidade Federal do Triângulo Mineiro (UFTM) em 1987-2017, agrupados em indivíduos com cardiopatia hipertensiva (HD) e sem cardiopatia (ND) e avaliados de acordo com fatores como idade, sexo (masculino e feminino) e cor (branco e não branco). A quantificação das fibras colágenas foi feita por morfometria computadorizada e a densidade mastocitária foi avaliada por métodos imuno-histoquímicos.
ResultadosHouve aumento significativo das fibras colágenas no ventrículo esquerdo no grupo HD em comparação com o grupo ND (p <0,001). Quanto à densidade mastocitária, houve aumento significativo no ventrículo esquerdo de indivíduos com HD imunomarcados por antiquimase/antitriptase (p=0,02) e também na densidade mastocitária no ventrículo esquerdo de indivíduos imunomarcados por antitriptase (p=0,03). Analisando o grupo de HD, houve uma correlação positiva e significativa entre a porcentagem de fibras colágenas no ventrículo esquerdo e a densidade mastocitária no ventrículo esquerdo imunomarcados por antiquimase/antitriptase (p=0,04) e também na densidade mastocitária no ventrículo esquerdo de indivíduos imunomarcados por antitriptase (p=0,02).
ConclusõesOs mastócitos estão envolvidos no desenvolvimento da cardiopatia hipertensiva, contribuem para a remodelação das fibras colágenas nessa doença.
Cardiovascular disease is the leading cause of mortality worldwide, and in Brazil is responsible for about 20% of all deaths in people over 30 years of age.1 There has been a change in recent years of the main causes of morbidity and mortality from infectious diseases to cardiovascular disease, cancer, and other chronic diseases, such as systemic hypertension (the epidemiological transition).2–5
One of the consequences of untreated systemic hypertension, which induces cardiac lesions and left ventricular (LV) hypertrophy, is hypertensive heart disease. A new component of the pathophysiology of hypertensive heart disease has been described: apoptosis of cardiomyocytes and changes in collagen synthesis and degradation, which may play an important role in the development of LV hypertrophy and myocardial injury.6–8
Studies have shown that chronic LV overload increases the density of cardiac mast cells, which when activated release a range of potent inflammatory and pro-fibrotic mediators that play an active part in cardiac remodeling.9,10
The mechanisms underlying progression of collagen deposition and fibrosis as a result of the presence of cardiac mast cells have not been fully elucidated. Therefore, the purpose of this study was to assess the formation of collagen fibers and mast cell density in hypertensive heart disease, in order to improve our understanding of the pathological processes involved.
HypothesisWe hypothesize that mast cells are involved in the development of hypertensive heart disease, contributing to the remodeling of collagen fibers in this disease.
BackgroundExperimental studies have reported that cardiac mast cells are involved in myocardial fibrosis. The first to describe this association were Olivetti et al.,11 who observed an increase in cardiac mast cells in the right ventricle of rats with pulmonary artery stenosis, although they did not indicate whether the mast cells were associated with fibrosis. Panizo et al.12 first investigated mast cells in the left ventricle in systemic hypertension, and noted a strong correlation between increased mast cell density and increased collagen.
Activated cardiac mast cells release a number of potent inflammatory and pro-fibrotic mediators, including cytokines, chemokines, and proteases. They can be activated by immunoglobulin E, by factors and histamine released by neighboring macrophages or T lymphocytes, or by components of the complement system (C3a and C5a). Once activated, they initiate degranulation, which is the process of exocytosis of the components of their granules.13,14 Other factors that activate cardiac mast cells have not been clearly identified, but endothelin-1, reactive oxygen species, substance P and interleukin (IL)-33 appear to be involved in the process.10,15–17 The most abundant proteins stored in the secretory granules of mast cells are endopeptidases, of which the most important are tryptase and chymase. Both are serine proteases, but they differ in activity and in expression patterns.18,19 Mast cells maturing in microenvironments of different tissues may vary in the type and amount of tryptase and chymase expressed.
The physiological role of tryptase and chymase is still unclear and their activity is observed not in normal tissues, but in tissues with lesions. Tryptase is the predominant enzyme in the granules.20 Human mast cells that contain tryptase in their granules express protease-activated receptor 2 (PAR-2). In vitro studies have shown that pretreatment of these cells with the PAR-2 activating peptide upregulates IL-8 (CXCL8).21 Incubation of tryptase in endothelial cell cultures induces neutrophil migration, which is dependent on IL-8.22 Another study demonstrated that incubation of cardiac fibroblasts with tryptase resulted in collagen proliferation and synthesis.23 It has been shown that chymase is able to convert angiotensin I into angiotensin II24 and is probably involved in structural remodeling associated with diseases of the cardiovascular system.18
It is clear that mast cells play an important role in various cardiac diseases, but due to the complex composition of the secretory granules and the plasticity of their phenotype, their contribution remains to be clarified.25 Molecular studies have been proposed in order to characterize the key genes involved in the modulation of these pathological processes, but they are still in their early stages and their findings cannot as yet be used in clinical practice.26–30 Since mast cells express some peptidases at high levels, immunohistochemistry and immunoassays using antibodies directed against these enzymes are experimentally useful to assess numbers of mast cells, their activation sites and associated diseases.31
MethodsSample collectionFragments were collected from the middle third of the LV free wall from 30 individuals autopsied by two pathologists at the Clinical Hospital of the Federal University of Triângulo Mineiro (UFTM), between 1987 and 2017. The autopsy protocols were analyzed to obtain data including age, gender, race, heart weight and body weight. Samples from individuals aged 18 or older were selected and then divided into two groups: those with hypertensive heart disease (HD) (n=15) and those with no heart disease (ND) (n=15). Cases with an incomplete autopsy report and individuals with Chagas disease or any other heart disease were excluded from the study. The ratio of heart weight (g) to body weight (g)×100, used in the definition of cardiac hypertrophy, was calculated in all cases.32
Sample preparationThe LV fragments had been fixed in 10% formaldehyde. They were first dehydrated in alcohols of increasing concentration (70-100%), cleared in xylene and embedded in paraffin.33 They were then cut into 4-μm sections and slide 1 was stained with picrosirius red, counter-colored with hematoxylin, for quantification of collagen fibers.
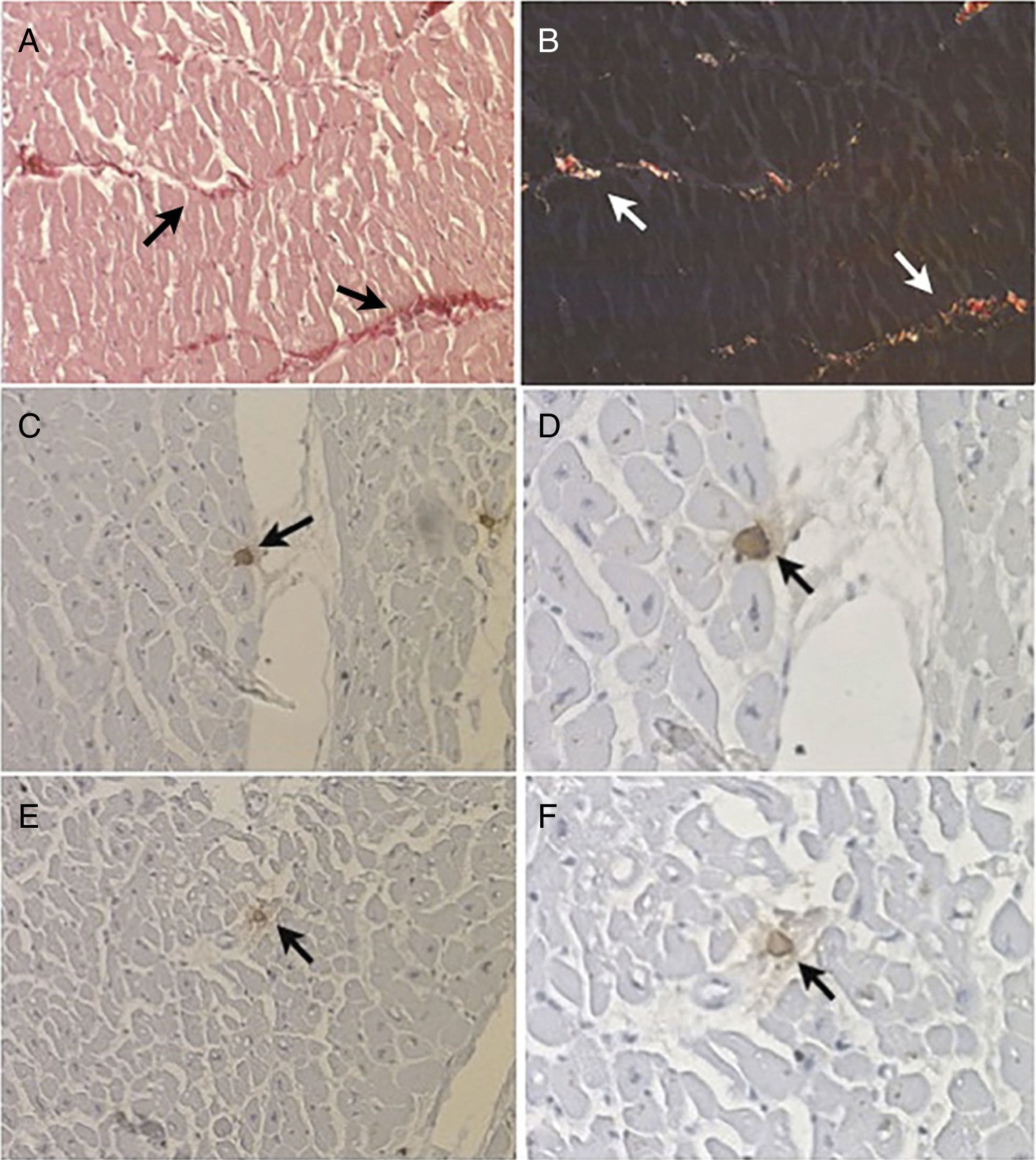
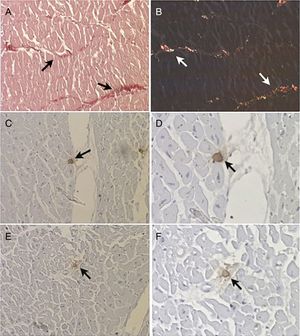
Morphometric analysis of collagen fibersThe number of fields for assessment and quantification of collagen fibers in the left ventricle was defined by the test of cumulative means.34 The histological section was divided into four quadrants and 40 fields of each fragment were digitized. Under polarized light the area of collagen fibers had a birefringent appearance, ranging from greenish yellow to reddish orange (Figures 1A and B). The collagen fibers were marked by the observer to obtain the percentage of collagen per field. The quantified image field was then scanned by a camera attached to the microscope. The morphometric analysis was performed using a LeicaQWin® Plus image analyzer (Leica Microsystems).
Histological section of a fragment of left ventricle examined (A) under visible light (arrows) (picrosirius stain, 200×) and (B) under polarized light, which reveals birefringent collagen fibers (arrows) (picrosirius stain, 200×); (C and D) mast cells (arrows) immunolabeled with anti-chymase antibodies (immunohistochemistry, 200×and 400×); (E and F) mast cells (arrows) immunolabeled with anti-tryptase antibodies (immunohistochemistry, 200×and 400×).
Slides 2 and 3 were used for immunohistochemical analysis to determine positivity to anti-human mast cell chymase antibodies (Diagnostic BioSystems®) diluted to 1:2000 (Figures 1C and D) and anti-human mast cell tryptase antibodies (Diagnostic BioSystems®) diluted to 1:500 (Figures 1E and F).35 Immunostained mast cells were quantified using a video camera connected to a conventional light microscope and to a computer with AxioVision® Rel 4.9.1 image analysis software (Carl Zeiss). Mast cells were quantified along the length of the section using a final magnification of 500×in a microscope field of 0.1520 mm2. The fragments were divided into four quadrants and, in each quadrant, ten randomly selected fields were quantified. The density of mast cells in each fragment was determined by dividing the total number of mast cells in the 40 quantified fields by the total area of the field, expressed in mast cells/mm2.18
Statistical analysisThe data were analyzed using GraphPad Prism® 5 software (GraphPad Software). The Shapiro-Wilk test was applied to test for normality of distribution of variables. The Student's t test (t) was used to compare groups for normally distributed variables and the Mann-Whitney U test for non-normal distributions. The correlation between two variables with normal distribution was analyzed using Pearson's test (r), and for those presenting non-normal variance, Spearman's test was used. Differences were considered statistically significant for p<0.05.
Ethical considerationsThis study was approved by the Research Ethics Committee of the Federal University of Triângulo Mineiro through protocol no. 1373.
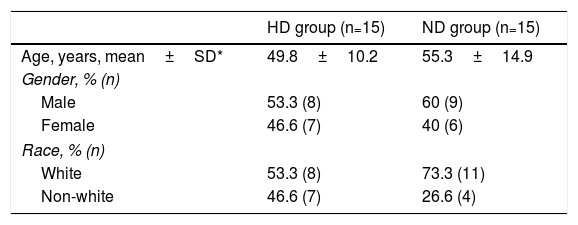
ResultsRegarding the general characteristics of the sample, the mean age of individuals in the HD group was 49.8±10.2 years, ranging from 44 to 66 years, and in the ND group the mean age was 55.3±14.9 years, ranging from 45 to 80 years. With regard to gender, 17 individuals (56.6%) were male and 13 (43.3%) were female. Considering race, 19 individuals (63.3%) were white and 11 (36.6%) were non-white. (Table 1).
Demographic characteristics of the sample of autopsied patients with hypertensive heart disease and without heart disease.
| HD group (n=15) | ND group (n=15) | |
|---|---|---|
| Age, years, mean±SD* | 49.8±10.2 | 55.3±14.9 |
| Gender, % (n) | ||
| Male | 53.3 (8) | 60 (9) |
| Female | 46.6 (7) | 40 (6) |
| Race, % (n) | ||
| White | 53.3 (8) | 73.3 (11) |
| Non-white | 46.6 (7) | 26.6 (4) |
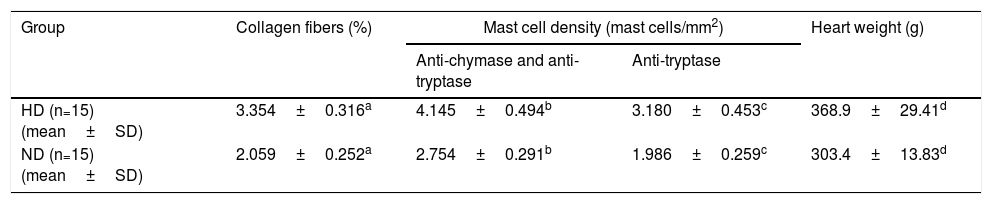
When the HD and ND groups were compared, a significantly higher percentage of collagen fibers in the left ventricle was seen in the HD group compared to the ND group (p=0.003). Mast cell density was also significantly higher in LV samples from individuals with HD immunolabeled with anti-chymase and anti-tryptase antibodies (p=0.02) and also in samples immunolabeled only with anti-tryptase antibodies (p=0.03). Heart weight was also significantly greater in HD compared to ND (p=0.05) (Table 2).
Comparison of percentage of collagen fibers, mast cell density and heart weight in individuals with hypertensive heart disease and without heart disease.
| Group | Collagen fibers (%) | Mast cell density (mast cells/mm2) | Heart weight (g) | |
|---|---|---|---|---|
| Anti-chymase and anti-tryptase | Anti-tryptase | |||
| HD (n=15) (mean±SD) | 3.354±0.316a | 4.145±0.494b | 3.180±0.453c | 368.9±29.41d |
| ND (n=15) (mean±SD) | 2.059±0.252a | 2.754±0.291b | 1.986±0.259c | 303.4±13.83d |
* p<0.05, Student's t test (t).
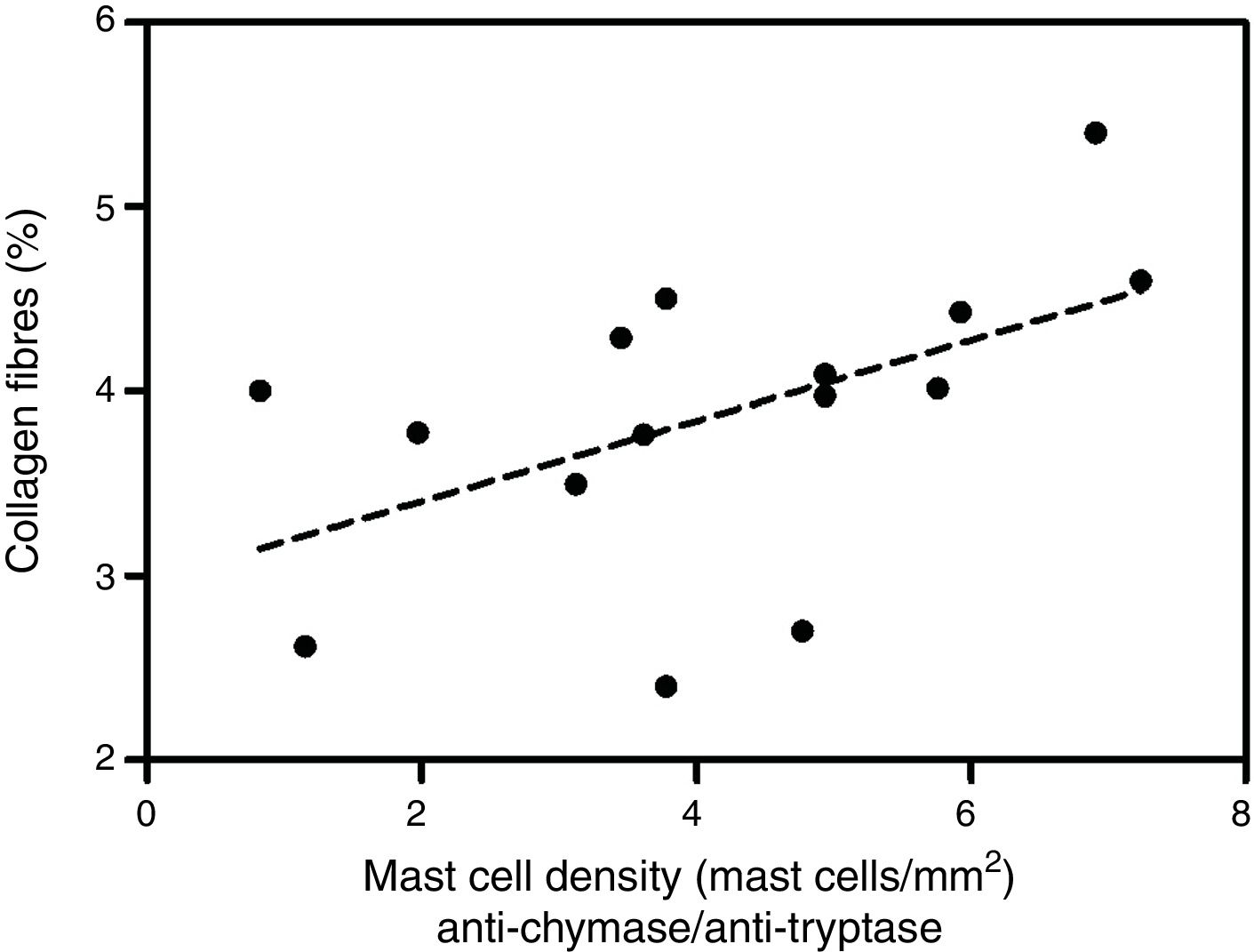
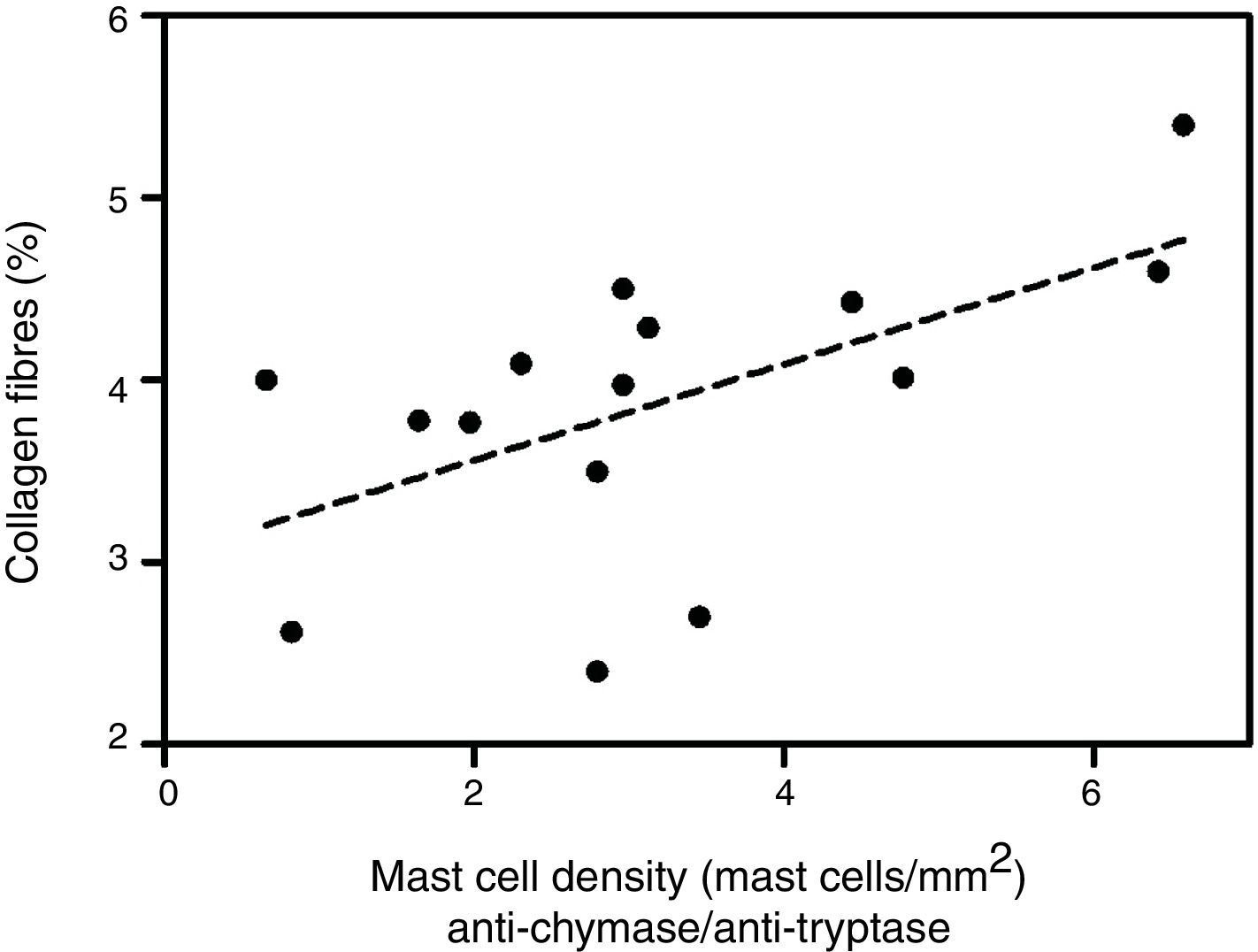
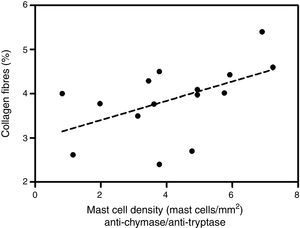
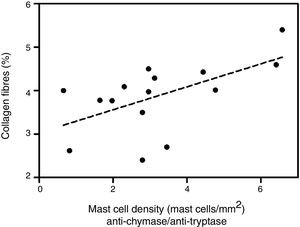
Analyzing the HD group, there was a significant positive correlation between the percentage of collagen fibers in the left ventricle and the density of mast cells immunolabeled with anti-chymase and anti-tryptase antibodies (r=0.517, p=0.04) (Figure 2) and also those immunolabeled only with anti-tryptase antibodies (r=0.574, p=0.02) (Figure 3). There was no significant correlation between the percentage of collagen fibers in the left ventricle and mast cell density in the ND group (data not shown).
There was no significant difference between percentage of collagen fibers, mast cell density and heart weight in terms of gender, race or age in either group.
DiscussionSystemic hypertension leads to structural and functional changes in the heart.36 In our study, we found an increase in collagen fibers in the HD group compared to the ND group. This finding is in line with the literature, which reports that fibrosis is among the morphological changes most often found in patients with hypertensive heart disease. It is characterized by diffuse or excessive accumulation of collagen fibers and increasing myocardial stiffness, which can lead to LV dysfunction and, eventually, heart failure.37–39 This reactive fibrosis is characteristic of pathological processes that trigger myocardial hypertrophy.6,40 The mechanisms responsible for the progression of LV hypertrophy include not only response to mechanical stress, but also the influence of neurohormones, growth factors and cytokines.41,42
Another study on the quantification of collagen in autopsied hearts of hypertensive and non-hypertensive individuals showed increased collagen in hypertensive patients with LV hypertrophy.43 This finding is in agreement with our research. Other studies have shown that type I collagen metabolism is increased in both humans and mice with hypertensive heart disease.44,45 Research has shown a close relationship between non-invasive histological diagnosis of myocardial fibrosis in animals and hypertensive patients and biochemical markers such as the carboxy-terminal propeptide of procollagen type I.37 Serum levels of this propeptide were higher in patients with hypertensive heart disease than in those with no heart disease,46 showing that the precursors of collagen synthesis are increased in individuals affected by hypertensive heart disease.
It has also been demonstrated that the ratio of serum matrix metalloproteinase (MMP)-1 and its tissue inhibitors is altered in patients with hypertensive heart disease,47 and that patients with reduced ejection fraction due to LV chamber dilatation present changes in the distribution of collagen fibers, mainly perivascular and scar, and considerable increases in MMP-1.40 These findings are related to the results of the present study, as activated MMPs are the main mediators of myocardial remodeling, mainly through the release of tryptase, which cleaves the pro-MMP-3 precursor, activating MMP-3 which then activates MMP-1, and through the release of chymase, which activates MMP-2 and MMP-9.48–53 This remodeling appears to be critical to the progression of LV hypertrophy.
In the present study, we found a significant increase in the density of mast cells in the left ventricle of individuals with hypertensive heart disease. Experimental studies that help explain our results demonstrated increased mast cell density in LV overload conditions and myocardial remodeling.16,23,50 The products of mast cell degranulation, besides activating the innate immune system, are pro-inflammatory, pro-fibrogenic and pro-hypertrophic. Among them are transforming growth factor beta (TGF-β), histamine, chymase, tryptase, IL-4 and tumor necrosis factor alpha,42,54–58 which correlates with the significant increase in collagen fibers in the left ventricle of patients with hypertensive heart disease. It is established in the literature that protein kinase C (PKC) is a convergence point for these events.59 Although in vitro studies have implicated PKC in mast cell degranulation, this has not been demonstrated in in vivo studies using heart failure models.60 In a study on hypertensive rats, it was demonstrated that inhibition of the PKC pathway attenuated mast cell degranulation without affecting mast cell density. This treatment also inhibited the infiltration of inflammatory cells, vasculopathy and fibrosis in the myocardium.61 Another study in rats with hyperlipidemia using Western blot analysis and immunohistochemical staining demonstrated that protein expression of TGF-β in the hearts of hyperlipidemic rats was significantly higher than in the control group.62
The lower percentage of collagen fibers and mast cell density in the left ventricle of women with hypertensive heart disease was not significant; however, this was also an expected result. The prevalence of prehypertension and hypertension in the literature is lower in women than in men.63 It appears that women suffer less effects from hypertension than men, due – among other factors – to the protective effects of endogenous estrogen.64 Bearing in mind the mean age of 54 years of the women analyzed, according to a study that reports that cardiac hypertrophy is more prevalent in menopausal women as a result of reduced levels of cardioprotective female sex hormones,65 these hormones also suppress the action of MMPs by reducing levels of cytokines such as TNF-α66 that control the activation of mast cells.
With regards to race, although non-white subjects had higher percentages of collagen fibers, the relationship between hypertensive heart disease and skin color is controversial. The ethnically mixed nature of the Brazilian population makes race a criterion of lesser validity for assessing the risk of developing heart disease.67 An autopsy study found that LV hypertrophy was not more prevalent in black patients,68 but in this study hypertension was an exclusion criterion, unlike our study, in which some subjects were hypertensive.
Studies have shown a relationship between severity and duration of hypertension.69 It has also been suggested that genetic control of ventricular mass may differ in different ethnic groups.27 Other studies have presented evidence to suggest that LV hypertrophy contributes to a higher frequency of sudden death, especially among black patients.70 Systemic hypertension is one of the main causes of disparities in mortality rates between blacks and whites in the USA, due to differences in prevalence and control. Other data suggest that primary care and social and environmental conditions reinforce this disparity in the prevalence of severe hypertension among blacks and whites.67,71,72
In our study, there was a non-significant increase in the percentage of collagen fibers with age. This finding may be due to the severity of systemic hypertension, since the mean age of the individuals analyzed in the study was 49.8 years in patients with hypertensive heart disease.73
ConclusionsOur study showed that, in patients with hypertensive heart disease, there is an increase in collagen fibers in the left ventricle. This reactive fibrosis is a fundamental component of the process of myocardial hypertrophy. The increase in the density of mast cells in the left ventricle of patients with hypertensive heart disease observed in the study indicates that mast cells have an important role in myocardial remodeling in hypertensive heart disease. Additional studies will help elucidate the mechanisms of collagen turnover, so that therapeutic targets may be determined in order to regulate the actions of the main proteases released in the activation of mast cells.
FundingThe present study was supported by the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq), Coordination of Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES), the Foundation for Research Support of the State of Minas Gerais (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG) and the Foundation Of Teaching and Research of Uberaba (Fundação de Ensino e Pesquisa de Uberaba, FUNEPU).
Conflicts of interestThe authors have no conflicts of interests to declare.