Coronary subclavian steal syndrome is an uncommon cause of ischemia recurrence after coronary artery bypass grafting. Endovascular treatment of subclavian artery stenosis or occlusion is increasingly common and appears to offer a safe and effective alternative to surgical revascularization. We report a case of recurrent angina after coronary artery bypass grafting for critical subclavian artery stenosis. The anomalous origin of the vertebral artery from the aortic arch was an indication for endovascular treatment. We discuss the diagnostic difficulties and the management pitfalls of subclavian artery angioplasty in this syndrome.
A síndrome de roubo da subclávia é uma causa incomum de recidiva de isquemia após a cirurgia de revascularização do miocárdio (CABG). A terapêutica endovascular da estenose ou oclusão da artéria subclávia é cada vez mais usada e parece oferecer uma opção segura e efetiva à revascularização cirúrgica. Apresentamos o caso de uma angina recorrente após CABG devida a estenose arterial crítica da subclávia. A origem anómala da artéria vertebral a partir da crossa da aorta favoreceu o tratamento endovascular. Discutimos as dificuldades diagnósticas e as dificuldades do tratamento da angioplastia da artéria subclávia durante essa síndrome.
Angina recurrence in a patient after coronary artery bypass grafting (CABG) is usually attributed to graft dysfunction; coronary subclavian steal syndrome (CSSS) is rarely mentioned. CSSS is defined by retrograde blood flow from the left internal mammary artery (LIMA) into the subclavian artery (SCA); it is related to proximal stenosis or total occlusion of the SCA.1 CSSS is not only difficult to diagnose but tricky to manage. Redux surgery is high risk in such patients and percutaneous treatment is sometimes made difficult by the angiographic appearance of the lesion. We report a case of CSSS and discuss diagnosis difficulties and management pitfalls.
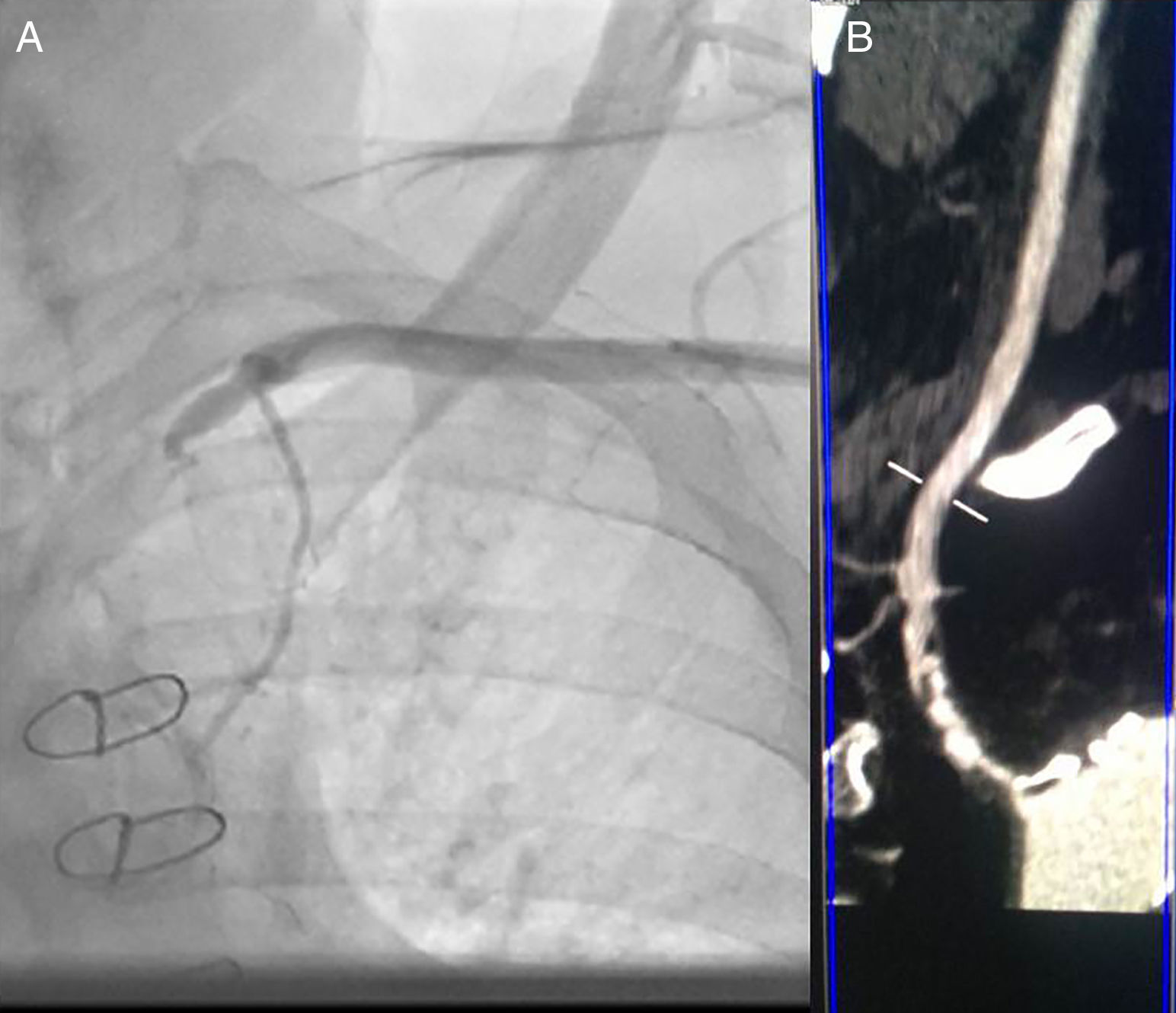
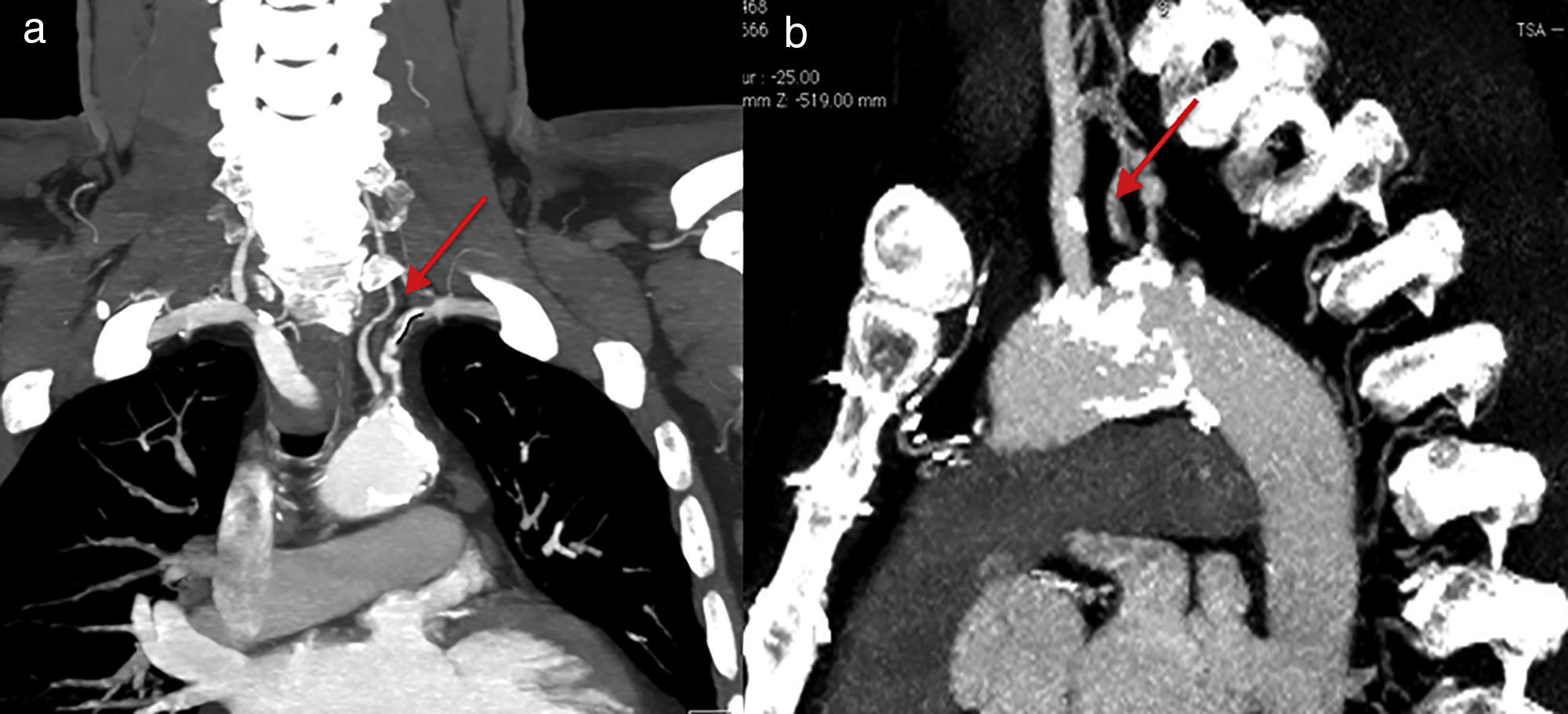
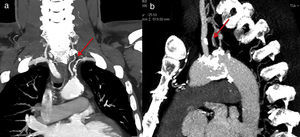
Case reportMr. M.K is a 55-year-old man with a medical history of hypertension, dyslipidemia, smoking, mild chronic renal disease and peripheral artery disease. In 2010, he had CABG of the left internal mammary artery (LIMA) to the left anterior descending (LAD) artery and a venous graft (VG) to the marginal. He remained asymptomatic for 6 years. Recently he developed chest pain on exertion, limiting his daily activities. He reported no other symptoms such as left arm claudication, paresthesia or dizziness. Physical examination was unremarkable, except for a difference in blood pressure between arms; left-arm pressure was 120/80 mmHg and right-arm pressure 140/80 mmHg. Left radial and brachial pulses were markedly reduced but we did not detect any abnormal murmur in the subclavian area. Electrocardiogram showed negative T waves in anterior leads. Echocardiography revealed a low left ventricular ejection fraction (LVEF=35%) with an anterior hypokinesia. Positron emission tomography (PET) showed anterior ischemia and inferior necrosis. A coronary angiogram was performed with initial right femoral access, showing a long severe stenosis in the LAD with competitive flow in the distal LAD and chronic total occlusions of the RCA and the first marginal. The saphenous-vein graft was also occluded. When trying to cannulate the LIMA, we failed to enter the subclavian artery. The aortogram revealed occlusion at the origin of the left SCA (Figure 1). We switched to left radial artery access; the LIMA was patent and free of stenosis. We decided to perform a transthoracic Doppler to check the flow in the LIMA, but the patient was lost to follow-up. Three months later, he returned with disabling angina. The transthoracic Doppler at that point showed retrograde flow in the LIMA, and confirmed CSSS. Computed tomographic angiography confirmed the presence of critical stenosis at the proximal SCA without any proximal stump (Figure 1); the aorta/clavicle junction was heavily calcified and the left internal carotid was very narrow with an ostial atheroma. Fortunately, the left vertebral artery originated directly from the descending aorta (Figure 2). After multidisciplinary team discussion and given the risk of redux surgery in a such patient, we opted for endovascular management. The procedure was not easy given the angiographic aspects of the risk of calcium embolism, the difficulty crossing the lesion and the proximity to the carotid artery. However, the vertebral artery originating from the descending aorta was a favorable factor for angioplasty.
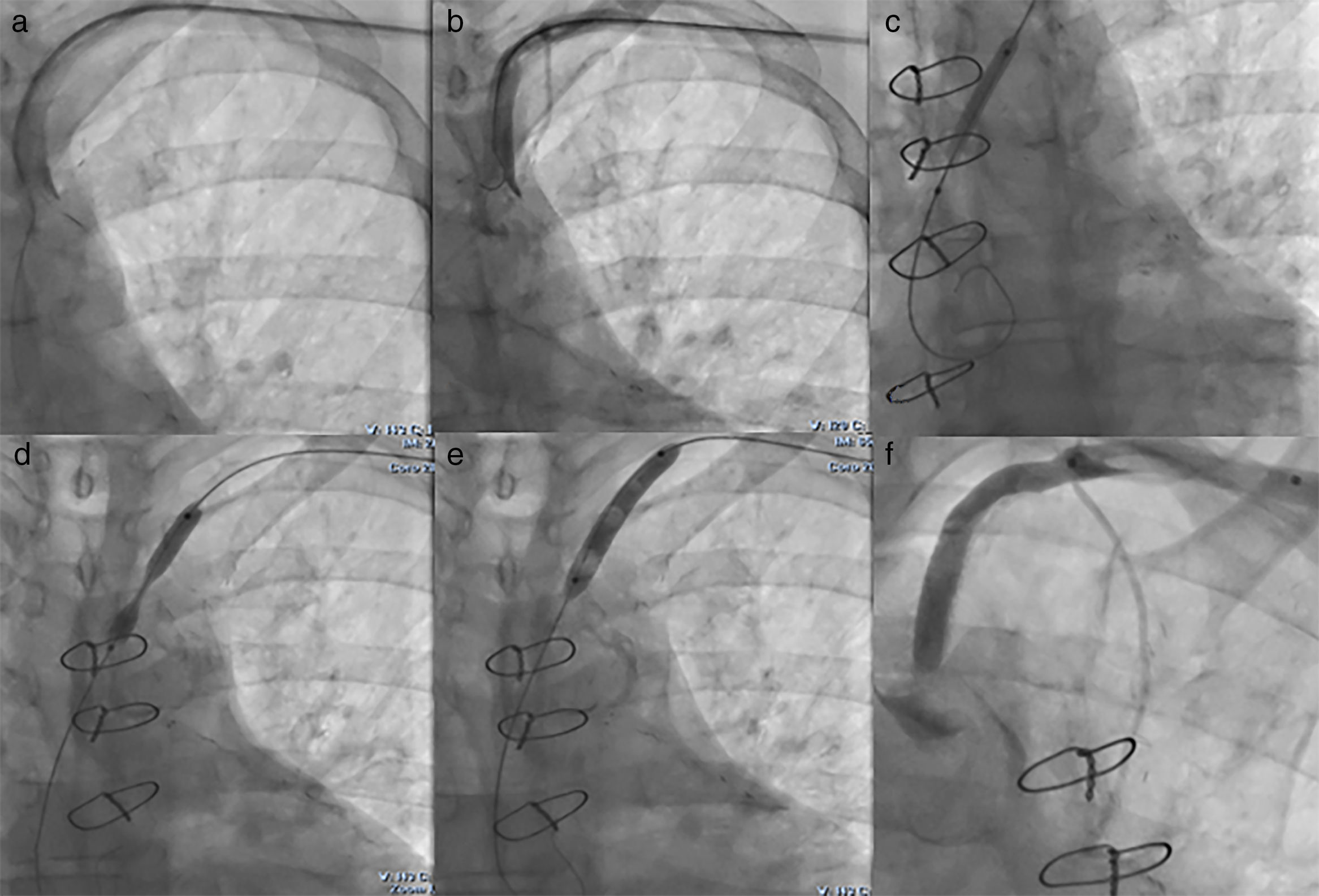
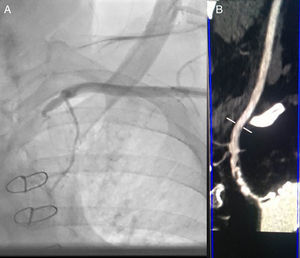
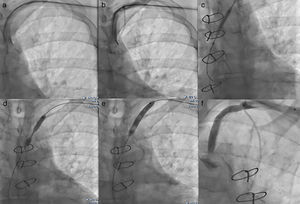
We performed the angioplasty of the SCA via a retrograde access from the left radial artery. The crossing of the lesion was laborious; we used many wires and even coronary wires (Whisper MS, Pilot 150 and Miracle 4) (Figure 3). Finally, a TIF Tip™ 0.018 Terumo Hydrophilic Guidewire was advanced through the catheter and the SCA occlusion was barely crossed, using a retrograde subintimal dissection. Predilation using a coaxial balloon (Admiral Xtreme, Medtronic, 4×40 mm) was performed to the nominal diameter after confirming the intravascular position (Figure 3). Next, a balloon expandable stent (6×37 mm) was inserted without complications. A final proximal optimizing post-stenting angioplasty was performed. The final angiography showed a good result with TIMI III flow in the LIMA (Figure 3). Immediately after the procedure, the patient had a normal radial pulse. Six months later, his LVEF had improved (LVEF=45%).
(a) First we crossed the lesion in a false lumen with a stiff guidewire with retrograde dissection of the aorta, so we withdrew the guidewire; (b) Attempt to cross the lesion with a coronary guidewire (Miracle 6); (c) Predilation of the lesion after the positioning of a TIF Tip™ 0.018 Terumo Hydrophilic Guidewire in subintima; (d) Release of the stent at the stenosis of the subclavian artery with 1 cm into the aorta; (e) Postdilation of the stent; (f) Final result with no residual stenosis and TIMI III flow of left internal mammary artery.
Nowadays, the LIMA graft is the most used as a result of the long-term patency and the low operative mortality rates.2 CSSS is an uncommon complication and has a low reported incidence (0.2% to 6.8%) after CABG surgery with the LIMA1,3,4; the first case was described in 1966.5 However, it seems to be underestimated and its incidence is currently rising as a result of the increasing use of the LIMA for CABG surgery.1 CSSS is caused by stenosis or occlusion of the proximal SCA before the origin of the LIMA, resulting in reduced coronary flow and sometimes reversed flow. The consequence is myocardial ischemia.1 SCA stenosis may be present before CABG surgery or develop subsequently as a result of atherosclerotic disease progression.2 Thereby, the ischemic symptoms can develop immediately following the CABG surgery or up to 7-8 years later.2 It is typically associated with signs of vertebrobasilar insufficiency.1 Physical examination can reveal asymmetric upper-limb pulses and pressures as in our case or abnormal murmur in the subclavian area. The association of recurrence of ischemic signs and asymmetric systolic pressure after CABG should be suggestive of CSSS. This case highlights the importance of a systematic routine preoperative screening of SCA stenosis before CABG with either the LIMA or the right internal mammary artery, particularly in patients with multiple cardiovascular risk factors. SCA stenosis is typically diagnosed using continuous-wave Doppler ultrasonography on the LIMA; it will show reversed flow. Computed tomography, magnetic resonance imaging or angiography can be used as confirmatory tests for any suspected cases of subclavian steal. The traditional treatment consisted of surgical revascularization with extra-thoracic carotid-subclavian, subclavian-to-subclavian or axillo-axillary bypass grafting.6 It was associated with excellent long-term patency and low mortality rates.6 Currently, endovascular therapy including percutaneous transluminal subclavian artery angioplasty has emerged as a good alternative to surgery.7,8 One of the largest studies including 170 patients who underwent stenting of subclavian or innominate arteries reported a technical success rate of 98.3%, with 99.4% for stenotic lesions and 90.5% for occlusions. There were no procedure-related deaths and one stroke (0.6%). At long-term follow-up, 82% of all treated patients remained asymptomatic with a primary patency of 83% and a secondary patency of 96%.9 The complication incidence is low but complications can be lethal; in a series of 10 patients reported by Faggioli et al.,10 a SCA spiral dissection with LIMA occlusion occurred in one patient. Fortunately, the complication was resolved with prolonged ballooning of the SCA.
In our case, angioplasty was technically difficult, mainly as a result of the lesion angulation, the ostial location, tortuosity and heavy calcification. Moreover, the RCA, the marginal coronary and the venous graft were all occluded, and compromising the flow in the LAD/LIMA vessels, the only patent coronary axis, could therefore be lethal. The procedure was performed via a retrograde transradial subintimal dissection approach. The treatment consisted of transluminal angioplasty with balloon expandable stent placement after gentle balloon predilation. Some specific technical features associated with the SCA angioplasty need to be considered. First of all, ultrasound guidance is sometimes required for radial or brachial artery access because of the reduced flow within the artery making palpation of the pulse impossible.11 Secondly, SCA access can be through an anterograde femoral approach or a combined simultaneous anterograde and retrograde approach.8 In a recent report, Satti et al. described controlled subintimal access of SCA occlusion using anterograde or simultaneous anterograde and retrograde access, a wire escalation approach, and then balloon predilation only to the size required to advance the stent in order to minimize large dissection or vessel rupture.8 The need for distal embolic protection when these occlusions are reopened is subject to debate and has rarely been reported.11 The flow direction within the vertebral artery did not immediately change to antegrade but rather did so gradually over 20 seconds to several minutes; this delay in flow reversal therefore served as a protective mechanism against cerebral embolism during balloon angioplasty and direct stenting for SCA stenosis, and embolic protection may not be necessary. However, SCA stenting after predilation required distal embolic protection because the VA blood flow changed to the anterograde direction after predilation. In our case, the vertebral artery originated directly from the aortic arch and thus protected against embolism. However, it could exacerbate signs of myocardial ischemia as there is no substitution of the vertebral reversed flow.
ConclusionRecurrence of angina in patients who have undergone CABG should alert us to suspect CSSS. Diagnosis can be made from physical examination by discovering basilar syndrome and, above all, asymmetric arm pressure. The association with abnormal origin of the vertebral artery is exceptional and it may modify the clinical presentation. Endovascular angioplasty has become the treatment of choice, but a thorough analysis of the angiographic lesion is essential.
Conflicts of interestThe authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.