Aortic stenosis is the most prevalent type of valvular disease in Europe. Surgical aortic valve replacement (SAVR) is the standard therapy, while transcatheter aortic valve implantation (TAVI) is an alternative in patients at unacceptably high surgical risk. Assessment by a heart team is recommended by the guidelines but there is little published evidence on this subject. The purpose of this paper is to describe the experience of a multidisciplinary TAVI program that began in 2008.
MethodsThe heart team prospectively assessed 473 patients using a standardized approach. A total of 214 patients were selected for TAVI and 80 for SAVR. Demographic, clinical and procedural characteristics and long-term success rates were compared between the groups.
ResultsTAVI patients were older than the SAVR group (median 83 vs. 81 years), and had higher surgical risk scores (median EuroSCORE II 5.3 vs. 3.6% and Society of Thoracic Surgeons score 5.1 vs. 3.1%), as did the patients under medical treatment only. These scores were unable to assess multiple comorbidities. Patients’ outcomes were different between the three groups (mortality with SAVR 25% vs. TAVI 37.6% vs. conservative therapy 57.6%, p=0.001).
ConclusionsThe heart team program was able to select candidates appropriately for TAVI, SAVR and conservative treatment, taking into account the risk of both invasive treatments. The use of a prospective standardized heart team approach is recommended, but requires continuous monitoring to ensure effectiveness in a timely manner.
A estenose aórtica é atualmente a doença valvular mais prevalente na Europa. A substituição valvular aórtica cirúrgica (SVAC) é atualmente considerada a terapêutica de primeira linha, a implantação de válvula aórtica percutânea (VAP) é considerada uma opção em doentes com elevado risco cirúrgico. A avaliação dos doentes pelo Heart Team encontra-se preconizada pelas recentes guidelines publicadas de doenças valvulares, contudo existem poucas publicações acerca dessa temática. O objetivo deste manuscrito é descrever a experiência de um programa multidisciplinar VAP, iniciado em 2008.
MétodosO Heart Team avaliou de forma prospetiva e padronizada 473 doentes. Desses, 214 foram selecionados para VAP e 80 para SVAC. Os grupos foram comparados no que respeita às suas características demográficas, clínicas, de procedimento e quanto à sua evolução (mortalidade).
ResultadosO grupo VAP apresentou maior idade do que o grupo SVAC (mediana 83 versus 81 anos) e apresentou scores de risco cirúrgico mais elevados (mediana Euroscore II 5,3 versus 3,6% e STS 5,1 versus 3,1%), tal como o grupo de doentes apenas sob terapêutica médica. Esses scores não foram capazes de avaliar múltiplas comorbilidades. A mortalidade entre os três grupos apresentou diferenças com significado estatístico (SVAC 25% versus VAP 37,6% versus terapêutica conservadora 57,6%, p=0,001).
ConclusõesO programa Heart Team foi capaz de selecionar de forma adequada os doentes para as diversas estratégias terapêuticas tendo em conta o risco de ambos os procedimentos invasivos. Uma abordagem eficiente e standardizada pela Heart Team deve ser estimulada, necessitando de reavaliação continua.
Degenerative aortic stenosis (AS) is the most prevalent type of valvular disease in Europe, due to aging populations and increased survival.1,2 When it develops, there is a long asymptomatic period.1,2 Once symptoms occur, the prognosis of severe AS is dire; sudden death is frequent and survival rates are only 15-50% at five years.1,3
Surgical aortic valve replacement (SAVR) is the standard therapy for severe AS,1,2,4 increasing survival and improving quality of life.1 In patients at high surgical risk, transcatheter aortic valve implantation (TAVI) is a good alternative, with procedural success rates of over 90%.1,4–8
Assessment by a heart team is currently considered essential in the selection of patients, based on their risk profile and the technical suitability of TAVI.1,5,6,9 Despite its importance, there is a lack of published evidence on this subject. In addition, considering the complexity and high costs involved in TAVI, the resources involved need to be rationalized.10
The aim of this paper was to assess a prospective standardized decision process by a heart team regarding patient selection and performance and results of the procedure, from the beginning of the program in 2008 until 2015.
MethodsFrom its beginning in 2008 up to 2015, the Hospital Santa Cruz TAVI program assessed 473 patients with severe symptomatic AS. A total of 383 patients completed the assessment process. Of these, 80 (20.9%) were selected for SAVR, 214 (55.9%) for TAVI and 89 (23.2%) for conservative treatment alone.
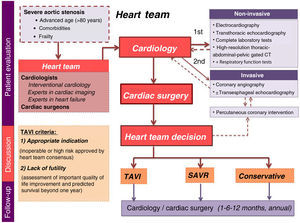
All patients are included in a prospective program that scrutinizes proposed candidates referred by their attending physicians. Briefly, the patient pathway starts with a clinical appointment that is followed by non-invasive and invasive tests and, finally, a multidisciplinary discussion (Figure 1). Candidate assessment is thorough, including the presence of symptoms, comorbidities and functional capacity. If needed, additional exams are requested: transthoracic echocardiography, complete laboratory tests including blood count, coagulation, N-terminal pro-B-type natriuretic peptide (NT-proBNP), kidney and liver function tests, and a high-resolution thoracic-abdominal-pelvic gated computed tomography (CT) scan. After complete assessment, the data are gathered and the patient is discussed at a dedicated heart team meeting to select the most appropriate therapeutic strategy. The team is composed of cardiologists – including clinical cardiologists, interventional cardiologists, and experts in cardiac imaging and heart failure – and cardiac surgeons, who meet fortnightly to discuss clinical cases. Results are continuously monitored for quality assessment.
The TAVI heart team criteria are based on two factors: indication (inoperable or high surgical risk, as approved by heart team consensus) and lack of futility (subjective assessment of significant improvement in quality of life and predicted survival beyond one year).
The possible access routes are determined and the final route is selected according to the first implantation date available in order to intervene as early as possible. Thus, an arterial approach is only considered when there is clinical or anatomical contraindication for any other route.
If the patient is approved for either of the invasive strategies (SAVR or TAVI), he or she goes on a waiting list for the procedure. Patients refused for any invasive intervention return to their referring physician to continue on optimal medical therapy. Those selected for SAVR or TAVI are followed at 30 days, six months and one year after the procedure, and then annually.
Statistical analysisAll clinical and procedural data are collected in a dedicated database (Cardiobase™) and a continuous ongoing registry, the Valve Catheter Restorative Operation on Santa Cruz Hospital (VCROSS). Anonymous data is exported for statistical analysis, performed with SPSS version 20.0 (IBM SPSS, Chicago, IL, USA).
Continuous variables are expressed as median (interquartile range [IQR]). Categorical variables are expressed as absolute value and respective percentages. Differences in means between groups were assessed by the Kruskal-Wallis test and differences between categorical variables were assessed by the chi-square test. A p-value of 0.05 or less was considered significant. Mortality during follow-up was analyzed by Kaplan-Meier survival curves.
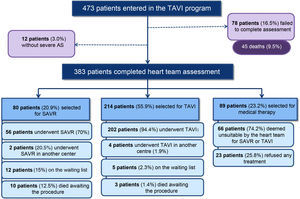
ResultsPatient selectionFrom 2008 up to 2015, 473 patients with severe symptomatic AS were assessed in the TAVI program at Hospital Santa Cruz (Figure 2).
As at April 2015, the assessment was not completed in 33 patients (7.0%) and 45 died during the initial assessment (9.5%). An additional 12 (3.0%) patients returned to their physicians because severe AS was not confirmed.
A total of 383 patients with severe AS completed the heart team assessment process. Of these, 80 (20.9%) were selected for SAVR, 214 (55.9%) for TAVI and 89 (23.2%) for medical treatment alone.
Population characteristicsThe characteristics of patients in the different treatment groups are shown in Table 1.
Characterization of patients treated by the different strategies.
| Variables, n (%) | SAVR (n=56) | TAVI (n=202) | Medical therapy (n=66) | p |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age in years, median (IQR) | 81 (76-84) | 83 (78-87) | 84 (80-88) | 0.023a |
| Male gender | 26 (46.4%) | 89 (44.1%) | 28 (42.4%) | 0.906b |
| Anthropometric data | ||||
| BMI in kg/m2, median (IQR) | 25.4 (22.9-29.3) | 25.8 (23.4-28.7) | 24.6 (23.3-27.7) | 0.435a |
| Comorbidities | ||||
| Hypertension | 40 (71.4%) | 157 (77.7%) | 51 (77.3%) | 0.609b |
| Diabetes | 13 (23.2%) | 71 (35.1%) | 19 (28.8%) | 0.199b |
| Coronary artery disease | 26 (46.4%) | 116 (57.4%) | 35 (53.0%) | 0.329b |
| Previous PCI | 14 (25%) | 86 (42.6%) | 16 (24.2%) | 0.005b |
| Previous CABG | 6 (10.7%) | 43 (21.3%) | 12 (18.2%) | 0.199b |
| Lung disease | 8 (14.3%) | 35 (17.3%) | 19 (28.9%) | 0.072b |
| PAD | 2 (3.6%) | 45 (22.3%) | 11 (16.7%) | 0.005b |
| Stroke | 1 (1.8%) | 33 (16.3%) | 16 (24.2%) | 0.002b |
| Porcelain aorta | 0 (0%) | 34 (16.8%) | 2 (3%) | 0.000b |
| Liver cirrhosis | 0 (0%) | 1 (0.5%) | 2 (3%) | 0.128b |
| Prior thoracic radiotherapy | 0 (0%) | 4 (2.0%) | 0 (0%) | 0.294b |
| CrCl (≤30 ml/min) | 7 (12.5%) | 46 (22.8%) | 17 (25.8%) | 0.167b |
| Left ventricular function | ||||
| Ejection fraction ≤40% | 4 (7.1%) | 36 (17.8%) | 18 (27.3%) | 0.015b |
| Surgical risk scores | ||||
| EuroSCORE I, median (IQR) | 9.8 (8.1-16.9) | 16.8 (11.4-25.7) | 18.4 (12.8-31.1) | 0.000a |
| EuroSCORE II, median (IQR) | 3.6 (2.5-5.5) | 5.3 (3.6-8.7) | 4.8 (3.8-7.6) | 0.005a |
| STS mortality, median (IQR) | 3.1 (1.8-5.2) | 5.1 (3.5-7.0) | 4.6 (2.8-6.4) | 0.000a |
BMI: body mass index; CABG: coronary artery bypass surgery; CrCl: creatinine clearance; IQR: interquartile range; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; STS: Society of Thoracic Surgeons score.
In all groups patients were mostly female. The medical therapy group had a higher median age than the other groups and patients undergoing SAVR were younger overall (p=0.023).
The most prevalent comorbidities were hypertension, diabetes and coronary artery disease. The TAVI group included a significantly higher number of patients previously treated by coronary angioplasty (p=0.005). The medical therapy group showed a higher prevalence of comorbidities such as lung disease, history of stroke and chronic kidney disease (stages 4 and 5). This group also presented a higher incidence of left ventricular dysfunction, followed by the TAVI group (p=0.015). Comorbidities such as porcelain aorta, liver cirrhosis and prior thoracic radiotherapy were only found in the TAVI and medical therapy groups, since these patients were considered inoperable.
Comparing the most used surgical risk scores, the medical therapy group had a higher median logistic EuroSCORE I (18.4%), followed by the TAVI group (16.8%) (p≤0.001), but the median values of both groups were below 20%. Mortality predicted by the EuroSCORE II and Society of Thoracic Surgeons (STS) scores was higher in the TAVI group, followed by the medical therapy group (p=0.005 and p<0.001, respectively). The SAVR group presented the lowest risk scores.
Patient selection for different treatmentsIn the eight years of the program, 207 TAVI procedures were performed in 202 patients. The procedure was successful in 97.6% of cases (Table 2). A total of five cases needed reintervention, mostly (n=3) due to prosthesis migration.
Success of TAVI procedure and causes of reintervention.
| Variable, n (%) | |
|---|---|
| Procedural success (n=202) | 197 (97.5%) |
| Causes of reintervention (n=5) | |
| Prosthesis migration to LVOT | 1 (20%) |
| Prosthesis embolization to aorta | 2 (40%) |
| Access failurea | 2 (40%) |
LVOT: left ventricular outflow tract.
The median time between the heart team's decision and bioprosthesis implantation was 70 days (IQR 34-129).
The most frequent implantation route was transfemoral (64.3%), followed by transapical (30.9%) (Table 3). The CoreValve® system was the most used device (58.0%), followed by the Edwards Sapiens® (39.1%). Table 4 describes the main characteristics of the percutaneous aortic valve implantation.
Implantation route and type of valve implanted in transcatheter aortic valve implantation procedures.
| Variable, n (%) | n=202 patients, 207 procedures |
|---|---|
| Implantation route | |
| Transfemoral | 133 (64.3%) |
| Transapical | 64 (30.9%) |
| Subclavian | 6 (2.9%) |
| Transaortic | 4 (1.9%) |
| Type of valve implanted | |
| Medtronic CoreValve® | 113 (54.6%) |
| Edwards Sapien XT® | 78 (37.7%) |
| St. Jude Portico® | 4 (1.9%) |
| Boston Lotus Valve® | 2 (1%) |
| Medtronic CoreValve Evolut® | 7 (3.4%) |
| Edwards Sapien 3® | 3 (1.4%) |
This table includes the first intervention and the five cases of reintervention.
Main characteristics of prosthetic aortic valves used in transcatheter aortic valve implantation procedures.
| Features | Sapiens XT Ascendra | Sapiens Certitude | Engager | Symetis Acurate | Sapiens XT Novoflex+ | Sapiens Commander | CoreValve | CoreValve Evolut R | Portico | Lotus |
|---|---|---|---|---|---|---|---|---|---|---|
| Implantation route | TA/TAo | TA/TAo | TA | TA | TF | TF | TF/TAo/Sc | TF/TAo/Sc | TF/Sc | TF |
| Time of preparation | Medium | Medium | Medium | Medium | Medium | Medium | Slow | Slow | Medium | Fast |
| Profile | 24-26F | 18F | 29F | 28F | 16-20F | 14-16F | 18F | 14F | 18F | 18F |
| Repositioning | No | No | Yes | No | No | No | No | Yes | Yes | Yes |
| Recapture | No | No | No | No | No | No | Yes | Yes | Yes | Yes |
| Release | Balloon | Balloon | SE | SE | Balloon | Balloon | SE | SE | SE | Mechanical |
| Pericardial tissue | Bovine | Bovine | Bovine | Porcine | Bovine | Bovine | Porcine | Porcine | Porcine + bovine | Bovine |
| Radiopaque marker | No | No | No | Yes | No | No | No | No | Yes | Yes |
Sc: subclavian; SE: self-expandable; TA: transapical; TAo: transaortic; TF: transfemoral.
Most of the patients selected for SAVR underwent isolated valvular intervention (85.7%). Only a minority also underwent associated coronary artery bypass surgery (14.3%).
Patients were refused for any kind of invasive intervention for a variety of reasons (Table 5). The most frequent reason was predicted lack of improvement of quality of life after the procedure due to comorbidities (39.4%), which included several patients with severe chronic lung disease. The second most frequent cause was life expectancy of less than a year (18.2%), in most cases due to diagnosed cancer at the time of TAVI assessment. The third most frequent reason was frailty (12.2%), an extreme vulnerability to adverse events as determined by the heart team, usually based on very low gait speed, severely decreased muscle mass and/or malnutrition. The fourth was the presence of associated severe disease of other valves making a major contribution to the patient's symptoms (all patients with concomitant severe mitral disease). Although patients were refused predominantly for clinical reasons, some were also rejected for technical reasons, including inadequate annulus size (3.0%) or the absence of a route for a TAVI procedure (1.5%).
Reasons given by the heart team for rejecting patients for surgical aortic valve replacement or transcatheter aortic valve implantation.
| Variable, n (%) | n=66 |
|---|---|
| Improvement of quality of life by TAVI unlikely because of comorbidities | 26 (39.4%) |
| Life expectancy <1 year | 12 (18.2%) |
| Frailty | 8 (12.2%) |
| Associated severe primary disease of other valves making a major contribution to the patient's symptoms | 6 (9.1%) |
| Significant clinical improvement after medical therapy or angioplasty | 4 (6.1%) |
| Severe left ventricular dysfunction, without contractile reserve | 3 (4.5%) |
| Inadequate annulus size (<18 mm) | 2 (3.0%) |
| Severe anemia, unable to receive blood transfusions if needed or contraindication to APT | 2 (3.0%) |
| Extreme left ventricular hypertrophy | 1 (1.5%) |
| No access for valve implantation | 1 (1.5%) |
| Active endocarditis | 1 (1.5%) |
APT: antiplatelet therapy; TAVI: transcatheter aortic valve implantation.
Note that a substantial proportion (25.8%) of all patients included in the medical therapy group (89 patients) refused any invasive therapy, either TAVI or SAVR (Figure 2).
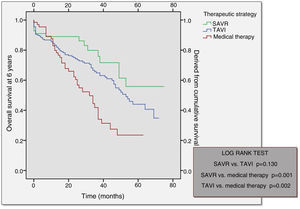
Long-term follow-upOver a median follow-up of 49 months (95% confidence interval 43-55), comparison of the different therapeutic strategies revealed higher mortality in the medical therapy group (57.6%) followed by the TAVI group (37.6%) (Table 6). Mortality at 30 days post-procedure (SAVR vs. TAVI) was almost the same in both groups, slightly higher in the SAVR group.
Mortality in the different treatment groups.
| SAVR (n=56) | TAVI (n=202) | Conservative therapy (n=66) | p | |
|---|---|---|---|---|
| Total mortality | 14 (25%) | 76 (37.6%) | 38 (57.6%) | 0.001 |
| Mortality 30 days | 4 (7.1%) | 9 (4.4%) | - | - |
| Mortality 30 days-1 year | 2 (3.6%) | 23 (11.4%) | 13 (19.7%) | - |
| Mortality 1-3 years | 3 (5.4%) | 26 (12.9%) | 19 (28.8%) | <0.00111 |
| Mortality 3-5 years | 5 (8.9%) | 16 (7.9%) | 6 (9.1%) | 0.001 |
| Mortality >5 years | - | 2 (1%) | - | - |
SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation.
Analysis of the Kaplan-Meier curves for the different therapeutic strategies shows that after an initial stage in which the surgical procedure and TAVI had higher periprocedural mortality, both subsequently depart from the medical therapy survival curve (Figure 3). Although SAVR appears to present a more favorable survival curve, there was no statistically significant difference in comparison with TAVI (p=0.130). Survival curves for both TAVI and SAVR were significantly different from that of the medical strategy.
DiscussionThe main contribution of this study is to provide evidence that implementation of a heart team, as recommended by the guidelines for treatment of severe aortic stenosis, should be performed prospectively under a well-defined, ongoing and carefully staged protocol that enables the activation of a multidisciplinary team that can provide all available therapies (TAVI, SAVR and conservative treatment).
Our group foresaw that patient selection would have a crucial role in the success of transcatheter aortic valve implantation, and thus a visionary multidisciplinary program was set in place. The heart team has become fundamental in the assessment of patients based on their risk profile and the technical suitability of TAVI.1,4–6,8,9,11–16 In the current context of an increasing elderly population, the availability of new treatments for severe aortic stenosis requires multidisciplinary clinical expertise for the accurate diagnosis of heart valve disease and assessment of comorbidities and risks of intervention, in addition to the rationalization of resources, considering the complexity and high costs of TAVI.10 The technique should be performed in centers of excellence with a trained multidisciplinary heart team treating a minimum of 50 cases per year, appropriate technical facilities and a prospective registry for monitoring purposes.14
A broad group of specialists is required to assess these patients and to provide a rapid selection process, since short-term mortality in these patients is significant.2,14,15 Our experience reports 45 deaths during the assessment phase (9.5%) and 15 deaths in patients after complete assessment awaiting SAVR or TAVI (3.2%). This highlights the severity of the disease and shows the need for a faster assessment process and treatment. Our median time of 70 days between the decision to perform TAVI and implantation of the bioprosthesis seems excessive, and this recently prompted our center to expand its multislice CT scan facility, which hopefully will reduce this delay.
Multidisciplinary interaction between physicians is needed, enabling all the patient's biopsychosocial characteristics to be considered.10 The clinical cardiologist is responsible for patient selection and indication and for pre- and post-procedure follow-up.10 The interventional cardiologist is responsible for organizing the heart team and monitoring procedural performance,10 while the cardiovascular surgeon is responsible for performing transaortic and transapical procedures, assisting with the transfemoral approach, and treating possible complications.10 The echocardiographer is responsible for anatomical and functional characterization of the diseased valve, intraoperative assessment of correct positioning of the prosthesis, and monitoring of post-procedural complications.10 The heart team also includes other professionals such as anesthetists, nurses and physical therapists. The consensus nowadays is not to perform a TAVI procedure in sites lacking a heart team.10
TAVI is currently recommended in symptomatic patients with severe AS who are at high surgical risk or are considered ineligible for SAVR.1,6,8,13,14 Surgical risk has been traditionally assessed using scores such as EuroSCORE and STS. A logistic EuroSCORE I of over 20% and a STS mortality score of more than 10% have been proposed as indicators of high surgical risk (in combination with clinical judgment).17 A EuroSCORE II cut-off of 7% appears to be equivalent to a Logistic EuroSCORE of 20% or an STS score of 10%.11 In our center, both the TAVI and medical therapy groups presented lower values than these. This variability of calculated scores has been described in other TAVI populations.9,11,18,19 A French center reported only half of patients reaching these threshold values.11 This is explained by the fact that these scoring systems were created on the basis of standard cardiac surgery databases and are thus unable to assess serious conditions that many of these patients suffer from.1,5,18,20,21 Comparison between the scores shows no significant differences in discriminatory power, and correlation between them appears to be at best modest.11,20 In the real world, the available surgical risk scores do not accurately predict mortality after TAVI.21 New promising and more accurate scores are emerging to replace the currently used surgical risk scores, including variables such as porcelain thoracic aorta, anemia, left ventricular dysfunction, recent myocardial infarction and critical aortic valve stenosis.22 For this reason, a heart team composed of individuals trained in systematic patient assessment enables better selection of individuals who may benefit from the various treatment strategies until more reliable risk scores become available.
In this cohort the main causes for patients being refused any kind of invasive procedure were low life expectancy and the presence of severe comorbidities which made TAVI unlikely to improve quality of life.1 One of these comorbidities is chronic obstructive pulmonary disease, which is associated with higher mortality at mid-term follow-up in patients undergoing TAVI.23 One study has revealed that TAVI was futile in more than one third of these patients and that a shorter distance in the six-minute walk test was a predictor of lack of benefit.23
Frailty was the third most common reason for patients being refused for TAVI in our center. Frailty syndrome is characterized by decreased muscle mass and energy expenditure and malnutrition, leading to extreme vulnerability to adverse events.6,24 A study assessing the frailty status of 159 patients through assessment of gait speed, grip strength, serum albumin and degree of independence in daily activities determined that frailty was not associated with increased periprocedural complications in patients selected for TAVI, but was associated with increased one-year mortality after the procedure.24 Our assessment of patients’ frailty was mostly subjective. This is a limitation, and the use of objective measurements and questionnaires has since been implemented.
Other comorbidities not included in current scores are liver cirrhosis, porcelain aorta and prior radiotherapy.1,4–6 Furthermore, assessment of neurocognitive function, functional status, mobility, and supports is increasingly being recognized as important in patient selection.8 The heart team is thus crucial in individualized patient assessment.1,5,6 This is particularly important in the current environment of limited resources.6 In the future a validated risk score for TAVI will probably be created, similar to the logistic EuroSCORE, EuroSCORE II and STS mortality score for surgical procedures, taking into account many variables already considered in the assessment process.5,6,18,22
Another important heart team decision is that of revascularization of coronary artery disease, which is often found coexisting with valvular disease, since they share the same risk factors.5,7 Up to three-quarters of patients undergoing TAVI have coronary artery disease.5 The optimal management of these patients is not well defined. Some authors argue that a staged approach (percutaneous coronary intervention followed by TAVI) is prudent in patients with lesions in the left main or proximal arteries (especially if dominant in the case of the right coronary artery or circumflex artery).5 Although this reduces procedure time, radiation and contrast exposure, it requires arterial access twice, with inherent risk for vascular and bleeding complications and additional hospitalization costs.7 In the elderly population with severe AS who undergo TAVI, coronary revascularization is usually incomplete.7 Judicious decisions concerning revascularization (not always deciding on complete revascularization) are more likely to achieve favorable mid-term outcomes.7
Anatomical assessment of patients is also essential, including arterial vasculature and the aortic valve apparatus (including the left ventricular outflow tract, aortic annulus diameter, sinus of Valsalva, sinotubular junction, ascending aorta and degree of calcification), to choose the most appropriate access route and transcatheter valve size.4–6,8,15 Which approach is used (transfemoral vs. transapical) has no prognostic value in acute and late outcomes.16 Usually the transfemoral approach is preferred, and an alternative route is only selected if there is a prohibitively small or diseased iliofemoral arterial system, mobile plaque, excessive calcification, or extreme tortuosity of the descending thoracic aorta.4,6,8,15 The fact that the most frequent implantation route was transfemoral (64.3%) reflects our strategy of implanting on the first available date without selecting a route if individually possible. This enabled the earliest treatment for each patient to be chosen.
The choice of prosthetic aortic valve is another important issue. As pointed out above, several devices are currently available with different characteristics that confer benefits in different clinical settings. For example, repositioning features are required for cases of extreme aortic calcification, potential coronary obstruction, bicuspid aortic valve, aortic regurgitation, valve-in-valve technique or left ventricular septal hypertrophy. Radiopaque markers are particularly useful in chronic kidney disease patients. The self-expandable feature is important when there is significant concomitant aortic regurgitation.
TAVI has been shown to be a feasible procedure.1,4–6,8 One-year survival rates of 60-80% are reported, depending on the severity of comorbidities.1,8 In our population the survival rate of TAVI patients at one-year follow-up was 84.1%. Mortality at 30 days was 4.4%, lower than the 9-10% usually described in other series.18
The literature on TAVI vs. SAVR for patients at high surgical risk shows that major adverse outcomes are similar between the two treatment modalities.3 However, the results are limited by inconsistent patient selection criteria, heterogeneous definitions of clinical endpoints and relatively short follow-up periods.3
As in our population, other studies show that after an initial stage in which the surgical procedure and TAVI had higher periprocedural mortality, both subsequently depart from the conservative therapy survival curve, showing a better outcome.19 Although SAVR appeared to present a more favorable survival curve, this was not statistically significant difference from that of TAVI, both having comparable survival curves at one year. SAVR performed in centers with a TAVI program is associated with significantly lower mortality and complication rates than that performed in centers without a TAVI program.25
A heart team approach is essential for TAVI in the current management of patients with severe aortic stenosis. This applies at each step of the procedure: patient selection, performance of the procedure, post-procedural care and evaluation of the results.26
ConclusionsA prospective multidisciplinary heart team program was able to appropriately select candidates for transcatheter aortic valve implantation, surgical aortic valve replacement and conservative treatment, matching the risk of both invasive treatments. The study is unique because it demonstrates the success of a standardized approach that requires continuous monitoring to be efficient in a timely manner.
Key pointsWhat is known about the topic?
- •
The literature available on TAVI vs. SAVR for patients at high surgical risk shows that major adverse outcomes are similar between the two treatment modalities. However, the results are limited by inconsistent patient selection criteria, heterogeneous definitions of clinical endpoints and relatively short follow-up periods.
- •
A heart team assessment is considered central to the selection of patients with severe AS. This applies at each step of the procedure: patient selection, performance of the procedure, post-procedural care and evaluation of the results.
- •
Despite its importance, there is a marked lack of publications in this area.
What does this study add?
- •
The heart team is crucial for individualized assessment of patients. This is particularly important in the current environment of limited resources.
- •
The main contribution of this study is to provide evidence that implementation of a heart team, as recommended by the guidelines for treatment of severe aortic stenosis, must be performed prospectively under a well-defined, ongoing and carefully staged protocol that enables the activation of a multidisciplinary team that can provide all available therapies (TAVI, SAVR and conservative treatment).
- •
This standardized approach requires continuous monitoring to be efficient in a timely manner.
The authors have no conflicts of interest to declare.