Clinical evidence on the bioresorbable magnesium scaffolds (BRS) is still scarce. We aim to assess clinical outcomes after magnesium BRS deployment in a real-world cohort of patients.
MethodsWe included in a non-randomized, prospective, single-center registry of all patients treated with at least one Magmaris device in our cath lab. Pre and postdilatation with optical coherence tomography guidance, as part of the 4Ps strategy, were performed in all cases. The primary endpoint was target lesion failure (TLF) at 12 months.
Results42 patients (with 42 lesions) underwent Magmaris percutaneous coronary intervention (PCI) between June 2016 to April 2017. PCI was performed in an acute setting in 54.76% cases; the most treated vessel was the anterior descending artery, with a mean diameter of 3.30±0.25 mm. All lesions underwent predilatation and postdilatation, with a mean postdilatation pressure of 19.2 atm. Procedural success rate was 100%. TLF rate was 4.7% at 12 months. None of our patients died or suffered myocardial infarction. Two patients (4.7%) underwent clinically-driven target lesion revascularization due to in-stent restenosis. No stent thrombosis was detected.
Conclusion12-months clinical outcomes after Magmaris PCI demonstrate its safety and feasibility when deployed in a 4Ps strategy.
A evidência clínica dos suportes vasculares restaurativos biorreabsorbíveis de magnésio é ainda escassa. O objetivo deste estudo consistiu na avaliação dos resultados clínicos após a implantação dos SVR (suportes vasculares restaurativos) de magnésio numa coorte de doentes do mundo real.
MétodosIncluímos num registo não aleatório, prospetivo e unicêntrico todos os doentes tratados com pelo menos um dispositivo Magmaris, no nosso laboratório de hemodinâmica. A pré e a pós-dilatação, sob a orientação de TCO como parte da estratégia dos 4 Ps, foram efetuadas em todos os casos. O objetivo primário foi a falência da lesão alvo (FLA) aos 12 meses.
ResultadosForam submetidos 42 doentes (com 42 lesões) a ICP com Magmaris entre junho de 2016 e abril de 2017. A ICP foi efetuada num contexto agudo em 54,76% dos casos, sendo a artéria descendente anterior alvo do maior número de tratamentos, com um diâmetro médio de vaso de 3,30±0,25 mm. Todas as lesões foram submetidas a pré e pós-dilatação, com uma pressão média de pós-dilatação de 19,2 atm. A taxa de sucesso do procedimento foi de 100%. A taxa da FLA foi de 4,7% aos 12 meses. Nenhum dos doentes faleceu ou sofreu enfarte do miocárdio. Dois doentes (4,7%) foram submetidos a revascularização da lesão alvo devido a restenose intra stent. Não foi detetada trombose de stent.
ConclusãoOs resultados clínicos a 12 meses após a ICP com Magmaris demonstraram segurança e viabilidade quando desenvolvidos sob uma estratégia de 4 Ps.
Bioresorbable scaffolds (BRS) first appeared more than 10 years ago and they were a revolution in interventional cardiology as they avoided permanent caging of the vessel. The first commercially available BRS was the Absorb® device (Abbot, Santa Clara, California, USA). Several randomized trials and real-world registries performed with Absorb® have provided important short and long-term clinical and angiographic outcomes. In fact, concerns about their safety emerged after detecting slightly higher rates of very late stent thrombosis in comparison to the standard metallic drug-eluting stents (DES).1,2 A common class effect was initially hypothesized for the Magmaris® device. However, results from BIOSOLVE II and III trials3–5 demonstrated no scaffold thrombosis in long-term follow-up of 12-24 months. Although these results are promising, there is still limited evidence from randomized trials. To gain a sound understanding of the behavior of Magmaris®, we present 12-month clinical outcomes from a real-world cohort of patients treated with the Magmaris® device in our cath lab.
MethodsStudy populationA prospective, all-comers registry was designed to include all patients treated with at least one Magmaris® device in our cath lab from June 2016 to April 2017.
Based on anatomical features, lesions considered as suitable for Magmaris® percutaneous coronary intervention (PCI) were de novo coronary lesions, measuring between 2.5 and 3.5 mm in diameter and with no/mild calcification. There were no restrictions based on lesion length. Angiographic exclusion criteria included left main disease, ostial lesions, chronic total occlusions and in-stent restenosis. All indications for PCI were accepted, including stable coronary artery disease and acute coronary syndromes (ACS). Being of a young age was a major inclusion criteria as patients with long life-expectancy could benefit the most from the favorable properties of BRS. Clinical exclusion criteria included past history or high risk of bleeding, heparin or antiplatelet treatment intolerance or expected survival <1 year.
All patients signed an informed consent form.
Device characteristicsMagmaris® BRS is currently the only available CE-mark approved metallic bioresorbable scaffold.6 This sirolimus-eluting BRS has a metallic bioresorbable backbone made from magnesium-alloy which is coated by a poly-l-lactic acid layer where the antiproliferative drug is embedded.3,6 Due to the mechanical properties of magnesium alloy, higher tensile strength and longer percentage elongation-at-break have been proposed for this BRS compared to polymeric scaffolds.6,7 In addition to this, electropolishing of magnesium-alloy backbone produces a smoother scaffold surface with rounded edges, which means better deliverability, trackability and pushability during PCI.8 A shorter time-to-resorption of 9-12 months has also been suggested for Magmaris® BRS, due to a two-steps degradation process, in which magnesium alloy is replaced by amorphous calcium phosphate.3,4
ProcedureOperators identified suitable lesions for Magmaris® deployment during PCI, according to the aforementioned criteria and current recommendations.7 In all cases, we performed a 4Ps strategy which includes: mandatory pre and postdilatation, proper sizing of vessel evaluation with intracoronary analyses and appropriate patient selection. Accordingly, optical coherence tomography (OCT) analyses were performed to obtain a precise assessment of the vessel diameter and to characterize the lesion. Predilatation with a semi-compliant balloon was mandatory to ensure good lesion preparation before scaffolding. High pressure postdilatation was also performed in all cases according to protocol, with non-compliant balloons in a 1:1 ballon/scaffold ratio or up to 0.5 mm longer. Additional OCT evaluations were performed, after scaffold deployment or postdilatation, to assess scaffold apposition and expansion and to quantify residual stenosis, if present.
Planned scaffold overlapping was not recommended. In the event of more than one scaffold being necessary to guarantee complete lesion coverage, the Magmaris® device should be considered. The implantation should be performed edge-to-edge, avoiding overlapped segments or gaps between devices.
All procedures were carried out while the patients were on unfractionated heparin treatment. At discharge, dual antiplatelet therapy was mandatory with aspirin and a P2Y12 inhibitor (ticagrelor, prasugrel or clopidogrel), according to current guidelines.9
Endpoints and definitionsThe primary endpoint was the target lesion failure (TLF) rate at 12-month follow-up, defined as the composite endpoint of cardiac death, target-vessel related myocardial infarction (MI) and clinically-driven target lesion revascularization (TLR).
Secondary endpoints were a) the global major adverse cardiac events (MACE) rate and b) scaffold thrombosis rate at 12-months.
TLR was defined as any repeat revascularization in the previously treated coronary segment or 5 mm proximally or distally. MACE was defined as cardiac death, any myocardial infarction and any coronary revascularization.
Procedure success was defined as final residual stenosis <20% in the absence of death, MI or TLR during in-hospital stay.
StatisticsContinuous variables are expressed as mean and standard deviation while categorical variables are presented as a percentage. Statistical analyses were performed using SPSS software, version 21.
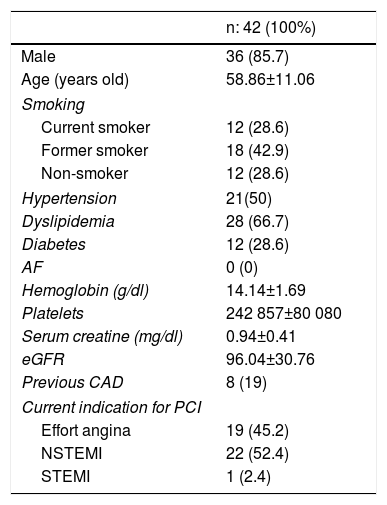
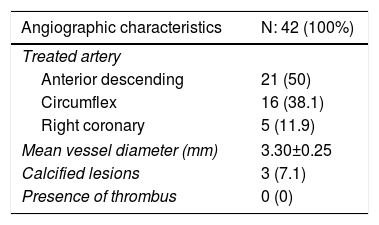
ResultsA total of 42 patients with 42 lesions were treated with Magmaris® scaffold in our cath lab between June 2016 and April 2017. Most of the patients were men; their mean age was 58 and they had several cardiovascular risk factors. 71.4% were former smokers, 50% had hypertension and 66.7% dyslipidemia (Table 1). None of them suffered from chronic kidney disease or anemia. 54.76% of the PCI were performed in an acute setting, 22 patients presenting with non-ST-elevation MI and one with ST-elevation MI. Angiographic and procedural data are shown in Table 2. The most frequently treated vessel was the anterior descending artery, with a mean vessel diameter of 3.30±0.25 mm. All lesions underwent predilatation, with a mean pressure of 15.3 atm. In most cases (37 out of 42), a single Magmaris device was deployed. Mean scaffold diameter and length were 3.30±0.25 and 20 mm, respectively. 100% of the lesions treated underwent high pressure postdilatation, with a mean pressure of 19.2 atm up to a maximum of 28 atm. Procedural success rate was 100%.
Baseline clinical characteristics.
| n: 42 (100%) | |
|---|---|
| Male | 36 (85.7) |
| Age (years old) | 58.86±11.06 |
| Smoking | |
| Current smoker | 12 (28.6) |
| Former smoker | 18 (42.9) |
| Non-smoker | 12 (28.6) |
| Hypertension | 21(50) |
| Dyslipidemia | 28 (66.7) |
| Diabetes | 12 (28.6) |
| AF | 0 (0) |
| Hemoglobin (g/dl) | 14.14±1.69 |
| Platelets | 242 857±80 080 |
| Serum creatine (mg/dl) | 0.94±0.41 |
| eGFR | 96.04±30.76 |
| Previous CAD | 8 (19) |
| Current indication for PCI | |
| Effort angina | 19 (45.2) |
| NSTEMI | 22 (52.4) |
| STEMI | 1 (2.4) |
AF: atrial fibrillation; CAD: coronary artery disease; eGFR: estimated glomerular filtration rate; NSTEMI: non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction.
Angiographic and procedural data.
| Angiographic characteristics | N: 42 (100%) |
|---|---|
| Treated artery | |
| Anterior descending | 21 (50) |
| Circumflex | 16 (38.1) |
| Right coronary | 5 (11.9) |
| Mean vessel diameter (mm) | 3.30±0.25 |
| Calcified lesions | 3 (7.1) |
| Presence of thrombus | 0 (0) |
| Procedural aspects | |
|---|---|
| Predilatation | 42 (100) |
| Mean balloon diameter (mm) | 3.29±0.25 |
| Mean pressure (atm) | 15.26±2.46 |
| Mean number scaffolds per lesion | 1.1 |
| Mean scaffold diameter (mm) | 3.30±0.25 |
| Mean scaffold length (mm) | 19.36±3.55 |
| Mean scaffolded length per lesion (mm) | 21.66 |
| Postdilatation | 42 (100) |
| Mean balloon diameter (mm) | 3.30±0.25 |
| Mean pressure (atm) | 19.21±3.98 |
| Maximal pressure (atm) | 28 |
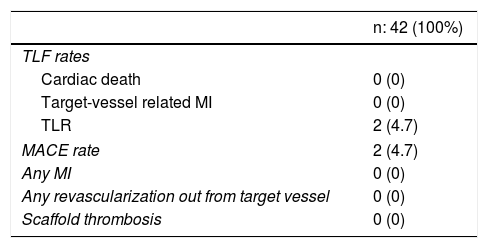
The 12-month TLF rate was 4.7% due to clinically-driven TLR in two patients who presented with effort angina and in-stent restenosis. No cardiac death or MI was reported (Table 3). No stent thrombosis was detected.
Clinical outcomes at 12-month follow-up.
| n: 42 (100%) | |
|---|---|
| TLF rates | |
| Cardiac death | 0 (0) |
| Target-vessel related MI | 0 (0) |
| TLR | 2 (4.7) |
| MACE rate | 2 (4.7) |
| Any MI | 0 (0) |
| Any revascularization out from target vessel | 0 (0) |
| Scaffold thrombosis | 0 (0) |
MACE: major adverse cardiac events; MI: myocardial infarction; TLF: target lesion failure; TLR: target lesion revascularization.
In this study we report the 12-month clinical outcomes after Magmaris®-PCI in a real-world cohort of patients.
The main findings include a) an appropriate acute performance of the device that allows the operator to achieve a 100% procedural success rate, with no in-hospital complications; b) optimal medium term outcomes with low TLF rates, and c) no stent thrombosis.
Clinical evidence on magnesium BRS is still limited. In the BIOSOLVE II and III trials,3,4 procedural and device success rates were high.3 Importantly, inadequate lesion preparation was the reason for device failure, in most of the cases. Taking this into account, experts recommend that Magmaris® deployment7 focuses on an exhaustive preparation of the lesion with almost mandatory predilatation and careful lesion and vessel assessment, preferably with an additional intracoronary imaging technique. Although a predilation, sizing and postdilation strategy was demonstrated to improve clinical and angiographic results for Absorb® PCI,10,11 there was no evidence to support this strategy for Magmaris®. However, based on our results, with a procedural success rate of 100% and favorable 12-month clinical outcomes when pre and postdilatation plus OCT guidance were applied, we strongly support the benefit of a 4Ps strategy for magnesium BRS PCI.
Recently published BIOSOLVE IV registry results12 corroborate those reported in our study, with 12-month TLF rates of 4.3% and 4.7%, respectively. BIOSOLVE IV results are consistent with those previously published in the BIOSOLVE II trial7 (TLF rates 3.4%) and also comparable to the TLR rates reported for metallic DES.13 However, some differences exist between BIOSOLVE IV and our registry. TLF rates in our study were due to two cases of in-stent restenosis in which clinically-driven TLR was necessary, with no target-vessel related MI. In the BIOSOLVE IV registry, 0.8% patients suffered from target-vessel-related MI.12 It is remarkable that our favorable clinical outcomes are consistent with previously published results even when PCI was performed in a more challenging setting: a) 54.7% of patients included in our registry underwent PCI during an ACS; b) 28.6% were diabetic and c) longer lesions were treated, with a mean scaffolded segment per lesion of 21.6 mm. In contrast, lesion length was 12.6 mm in the BIOSOLVE II trial3 and 14.5 mm in BIOSOLVE IV registry.12 We suggest that these favorable outcomes result from optimal patient selection, where young patients, with non-calcified, type A de novo lesions could benefit the most from a Magnesium BRS PCI.
Concerns were raised in the scientific community about the safety of bioresorbable scaffolds when higher rates of late/very late scaffold thrombosis were detected in polylactic BRS when compared to metallic everolimus DES.1,2 However, results from the BIOSOLVE II trial demonstrate a different behavior for magnesium BRS, with no scaffold thrombosis (0%) detected in 24-months of follow-up.5 No stent thrombosis was also reported in a 12 months follow-up in our registry. We consider this a remarkable finding as more than half of the included patients (54.76%) underwent PCI during an ACS, which represents most thrombogenic scenarios for a PCI. This is an encouraging finding which differentiates magnesium BRS from everolimus-polylactic BRS, which was shown to have higher stent thrombosis rates in ACS-PCI.14
The main limitation of our study is the small number of patient enrolled in the study. More evidence is needed to confirm our findings in a larger population, along with randomized studies.
Conclusion12-month clinical outcomes for magnesium BRS PCI in a real-world cohort of patients demonstrate the safety and feasibility of the technique with no detection of scaffold thrombosis.
FundingThis research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.
DisclosuresThis study was conducted in accordance with the Declaration of Helsinki and written informed consent was obtained from all patients.