The true prevalence of chronic thromboembolic pulmonary hypertension (CTEPH) after pulmonary embolism (PE) in the Portuguese population remains unknown. We aimed to assess the prevalence and predictors of CTEPH two years after a symptomatic high- (HR) or intermediate-high risk (IHR) PE.
MethodsWe conducted a retrospective cohort study of patients admitted with PE between 2014 and 2019 to a Portuguese referral center for pulmonary hypertension.
ResultsIn this single-center registry of 969 patients admitted with PE (annual incidence of 46/100000 population), 194 had HR (5.4%) and IHR (14.7%) PE. After excluding patients who died or had no follow-up in the first three months, 129 patients were included in the analysis. The overall prevalence of suspected CTEPH by clinical assessment, Doppler echocardiography and V/Q lung scan was 6.2% (eight patients). CTEPH was confirmed by right heart catheterization in four of these (3.1%). Increased pulmonary artery systolic pressure (PASP) at admission (OR 1.12; 95% CI 1.04–1.22; p=0.005) and the presence of varicose veins in the lower limbs (OR 7.47; 95% CI 1.53–36.41; p=0.013) were predictors of CTEPH. PASP >60 mmHg at admission identified patients with CTEPH at follow-up with sensitivity and specificity of 83.3% and 76.3%, respectively. All patients diagnosed with CTEPH had at least two radiological findings suggestive of CTEPH at the index event.
ConclusionsIn our cohort, the prevalence of CTEPH in survivors of severe forms of acute PE was 6.2%. PASP above 60 mmHg and supporting radiological findings on the index computed tomography scan are highly suggestive of acute-on-chronic CTEPH.
A prevalência da hipertensão pulmonar tromboembólica crónica (HPTEC) após embolia pulmonar aguda (EP) na população portuguesa permanece desconhecida. O objetivo do nosso trabalho foi avaliar a prevalência e os fatores de risco de HPTEC dois anos após uma EP sintomática de alto risco (A) e risco intermédio-alto (IA).
MétodosEstudo de coorte retrospetivo que inclui doentes admitidos com EP entre 2014-2019 num centro de referência nacional para hipertensão pulmonar.
ResultadosDos 969 doentes admitidos com EP (incidência anual de 46/100.000 habitantes), 194 foram estratificados como EP de risco A (5,4%) e IA (14,7%). Após exclusão dos doentes que faleceram ou sem seguimento nos primeiros três meses, 129 doentes foram incluídos na análise. A prevalência geral de HPTEC suspeita por avaliação clínica, ecocardiograma com Doppler e cintigrafia pulmonar de ventilação/perfusão foi de 6,2% (8 doentes). HPTEC foi confirmada por cateterismo cardíaco direito em quatro desses doentes (3,1%). A elevação da pressão sistólica da artéria pulmonar (PAPs) na admissão (OR 1,12; IC95% 1,04-1,22; p=0,005) e a presença de varizes nos membros inferiores (OR 7,47; IC95% 1,53-36,41; p=0,013) foram preditores de HPTEC. PAPs na admissão >60 mmHg identificou doentes com HPTEC no seguimento com valor de sensibilidade e especificidade de 83,3% e 76,3%, respetivamente. Todos os doentes diagnosticados com HPTEC tiveram pelo menos dois achados radiológicos sugestivos de HPTEC no evento índice.
ConclusõesNa nossa coorte, a prevalência de HPTEC nos sobreviventes de formas graves de EP aguda foi de 6,2%. Uma pressão arterial pulmonar sistólica acima de 60 mmHg e achados radiológicos de suporte na tomografia computadorizada do evento índice são altamente sugestivos da presença de HPTEC preexistente no momento do diagnóstico de EP aguda.
Chronic thromboembolic pulmonary hypertension (CTEPH), classified as Group 4 pulmonary hypertension,1,2 is one of the leading causes of pulmonary hypertension3,4 and a potentially curable major cause of morbidity and mortality,5 with a poor prognosis if left untreated.6
The cumulative incidence of CTEPH following symptomatic pulmonary embolism (PE) is estimated to be 0.1–9.1% two years after the acute event.3,7–11 Differences in patient selection criteria12,13 and study design may explain the discrepancies in the reported results, with higher prevalences found in prospective studies and in those not confirmed by right heart catheterization (RHC),12,14 the gold standard for CTEPH diagnosis.4 Small study populations and the low prevalence of CTEPH leading to scarcity of data also contribute to the high margins of error reported in studies.7,15,16
More severe pulmonary hypertension at the time of the PE event could suggest the presence of pre-existing CTEPH at the time of the initial PE diagnosis, which has been proposed as an explanation for the higher incidence in recent studies.17 However, this cannot be confirmed if hemodynamic assessment is not performed at the time of the initial acute PE.17
Estimation of CTEPH incidence and risk are of paramount importance for management decisions and for the establishment of standardized screening protocols after a PE event. To date, the true incidence and prevalence of CTEPH following PE in the Portuguese population remain unknown.
ObjectivesThe present study aimed: (1) to assess the prevalence of CTEPH two years after a symptomatic high- or intermediate-high risk acute PE in a Portuguese population; (2) to determine potential risk factors for CTEPH following an intermediate-high or high-risk PE; (3) to apply a CTEPH prediction score10,18 in a Portuguese population; and (4) to analyze initial computed tomography pulmonary angiography (CTPA) of the acute PE to identify patients with findings suggestive of CTEPH at the time of PE diagnosis (acute-on-chronic PE).
MethodsStudy populationThis retrospective cohort study conducted in a Portuguese referral center for pulmonary hypertension included all patients hospitalized with PE between 2014 and 2019, identified according to the International Classification of Diseases, Ninth (ICD-9) or Tenth (ICD-10) Revision, applying ICD-9 codes for “pulmonary embolism” (code 415.1) for the period between January 1, 2014 and December 31, 2015 and ICD-10 codes (I26.92, I26.99, I26.02, or I26.09) for patients hospitalized between January 1, 2016 and December 31, 2019. Only patients with a PE diagnosis confirmed by imaging techniques or autopsy were included in the study.
Through individual examination of each of the 969 clinical records identified with a PE diagnosis (including admission transthoracic echocardiography or CTPA and troponin levels when applicable), patient risk was stratified according to the criteria proposed by the current European Society of Cardiology and European Respiratory Society guidelines,3 except that the pulmonary embolism severity index (PESI) score was not considered in this stratification, and only hemodynamic stability, right ventricular dysfunction and troponin levels were used.
To calculate the incidence of episodes of hospitalization with PE, estimates of the resident population provided by Statistics Portugal (INE; www.ine.pt) of the corresponding geographic areas were obtained for the years under study. The incidence of PE in our population was estimated as the number of estimated new PE episodes per year and was expressed as 100000 population/year calculated as a six-year average.
Intermediate-high and high-risk PE were further analyzed for the purposes of this study, including estimation of CTEPH prevalence and determination of CTEPH predictors. Patients who died or were lost to follow-up within three months of the acute PE event were excluded from this further analysis. All patients included in this analysis had at least one follow-up appointment and were managed according to current guidelines.
Hospital clinical records (from hospital or emergency room admissions) and hospital or primary care appointments accessible from the national electronic registry (Registo de Saúde Eletrónico) provided data on demographics, comorbidities, clinical presentation, laboratory and imaging findings, treatment of PE and CTEPH, clinical course, mortality and risk factors for CTEPH. Follow-up was analyzed in all patients until the last available record or date of death.
The study protocol was approved by the institutional ethics committee. The protocol was established in accordance with the 1975 Helsinki Declaration of the World Medical Association. The corresponding authors are entirely responsible for the integrity of data and data analysis. After data collection individual participants were anonymized.
Outcome definitionsThe primary purpose of this study was to estimate the prevalence of CTEPH following a symptomatic intermediate-high or high-risk PE in a Portuguese population.
CTEPH was considered probable in patients, irrespective of symptoms, in the presence of persistent mismatched perfusion defects on ventilation/perfusion (V/Q) scan and intermediate- or high-probability pulmonary hypertension on echocardiography but not confirmed by RHC.2
CTEPH was considered confirmed when, in addition to the suggested changes on the V/Q scan, RHC established the diagnosis and excluded other forms of pulmonary hypertension. CTEPH was defined hemodynamically according to current guidelines1,2 as precapillary pulmonary hypertension with a mean pulmonary arterial pressure (mPAP) >20 mmHg, pulmonary arterial wedge pressure (PAWP) ≤15 mmHg and pulmonary vascular resistance (PVR) >2 Wood units, after at least three months of therapeutic anticoagulation.
In the present study, suspected CTEPH included both confirmed and probable CTEPH.
Secondary purposes were to identify CTEPH predictors and to analyze CTPA at the time of the initial PE event in order to identify patients with unknown pre-existing CTEPH at the time of the acute PE event (acute-on-chronic PE).
Potential predictors of CTEPH considered in the analysis included the following: age, PE admission risk, cardiovascular comorbidities, hormone therapy, previous deep venous thrombosis (DVT), previous PE, active malignancy, recent major surgery, immobility, prolonged travel, idiopathic PE, recurrent PE, symptom onset >2 weeks before PE diagnosis, thyroid hormone replacement, splenectomy, pacemaker lead infection, ventriculoatrial shunt, antiphospholipid syndrome, myeloproliferative disorder, varicose veins in lower limbs, PE presentation, PESI score, peak N-terminal pro-brain natriuretic peptide (NT-proBNP) at the time of the PE event, echocardiographic findings at PE diagnosis and during follow-up, CTPA findings at PE diagnosis, and PE treatment, particularly reperfusion.
The CTEPH prediction score18 was applied to the 129 patients included in the analysis. Analysis of its accuracy in excluding CTEPH in our population was further undertaken.
The CTPA of patients with suspected CTEPH at the initial event was retrospectively examined by an expert radiologist to identify findings suggesting the presence of unidentified CTEPH at the time of the initial PE event. These findings included the presence of mural thrombi lining the pulmonary vascular walls, eccentric wall-adherent filling defects, intravascular webs or bands, mosaic parenchymal perfusion patterns, right ventricular hypertrophy or right atrial dilatation, pericardial effusion, dilatation of the main pulmonary artery (>29 mm in men, >27 mm in women) and dilated bronchial arteries (≥1.5 mm).4,17,19,20
Statistical analysisContinuous variables were presented as mean±standard deviation or median and interquartile range (IQR) and were compared between groups using the independent samples t test or the Mann–Whitney U test, according to distribution. Normality was tested with the Shapiro–Wilk test and homogeneity of variances with Levene's test. Categorical variables were presented as frequencies and percentages and were compared using the chi-square test or Fisher's exact test, as appropriate. Logistic regression analysis was used to assess predictors of the presence of CTEPH during follow-up using the enter method. The Hosmer–Lemeshow test was used to calibrate the regression model. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. The discriminative ability of the previously published CTEPH prediction score10 to predict CTEPH in our population was assessed using receiver operating characteristic (ROC) curve analysis. All reported p values were two-sided and a p-value <0.05 was considered statistically significant. Statistical analysis was performed with IBM SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA).
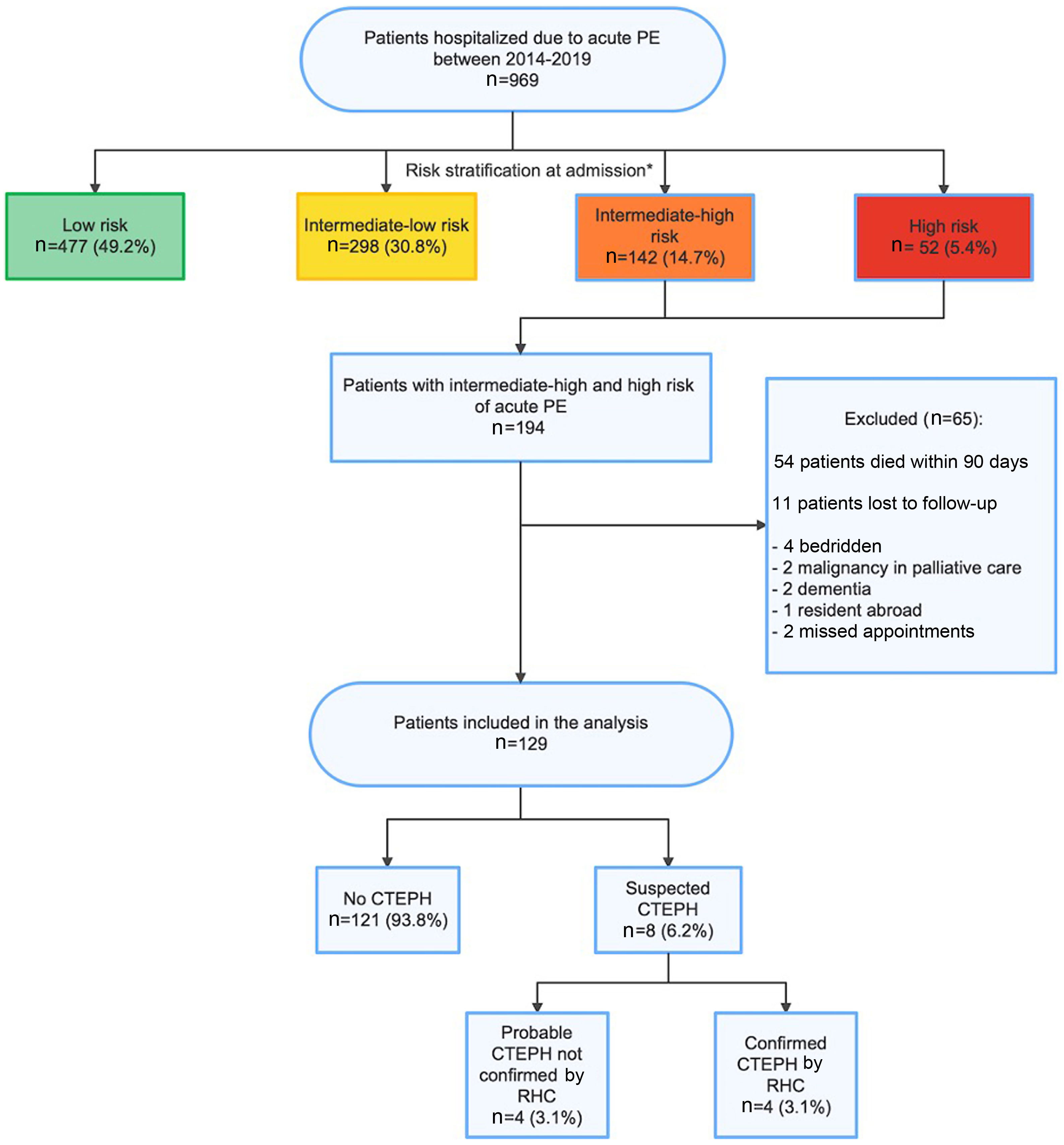
ResultsStudy populationBetween January 2014 and December 2019, 969 patients were hospitalized with PE, as depicted in the study flowchart (Figure 1). The mean annual incidence was estimated at 46/100000 population. Of these, 477 (49.2%) were stratified as low-risk patients, 298 (30.8%) as intermediate-low risk, 142 (14.7%) as intermediate-high risk and 52 (5.4%) as high-risk patients.
Study flowchart. * Risk stratification at admission following the current ESC guidelines, although PESI score was not considered for this categorization: low risk (absence of right ventricular [RV] dysfunction and troponin elevation); intermediate-low risk (RV dysfunction on transthoracic echocardiogram [TTE] or computed tomography pulmonary angiography [CTPA]; or elevated troponin levels); intermediate-high risk PE (RV dysfunction on TTE or CTPA and elevated troponin levels); high-risk (cardiac arrest: need for cardiopulmonary resuscitation; obstructive shock: hypotension (systolic blood pressure [BP] <90 mmHg) or vasopressors required to achieve BP >90 mmHg despite adequate filling status) and end-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria; increased serum lactate); persistent hypotension (systolic BP <90 mmHg or systolic BP drop >40 mmHg, lasting longer than 15 min and not caused by new-onset arrhythmia, hypovolemia, or sepsis). CTEPH: chronic thromboembolic pulmonary hypertension; PE: pulmonary embolism; RHC: right heart catheterization.
Of the 194 patients included in the study with intermediate-high or high-risk PE, 54 were excluded due to death in the 90 days following the PE event. Of the 140 patients alive at least 90 days after the PE event, 11 were not included due to loss of clinical follow-up in the first three months. Therefore, the final study population included 129 patients, with a median age of 69.0 years (56.0–79.0) at the time of the first PE event. The median follow-up was 41.0 (24.0–58.5) months.
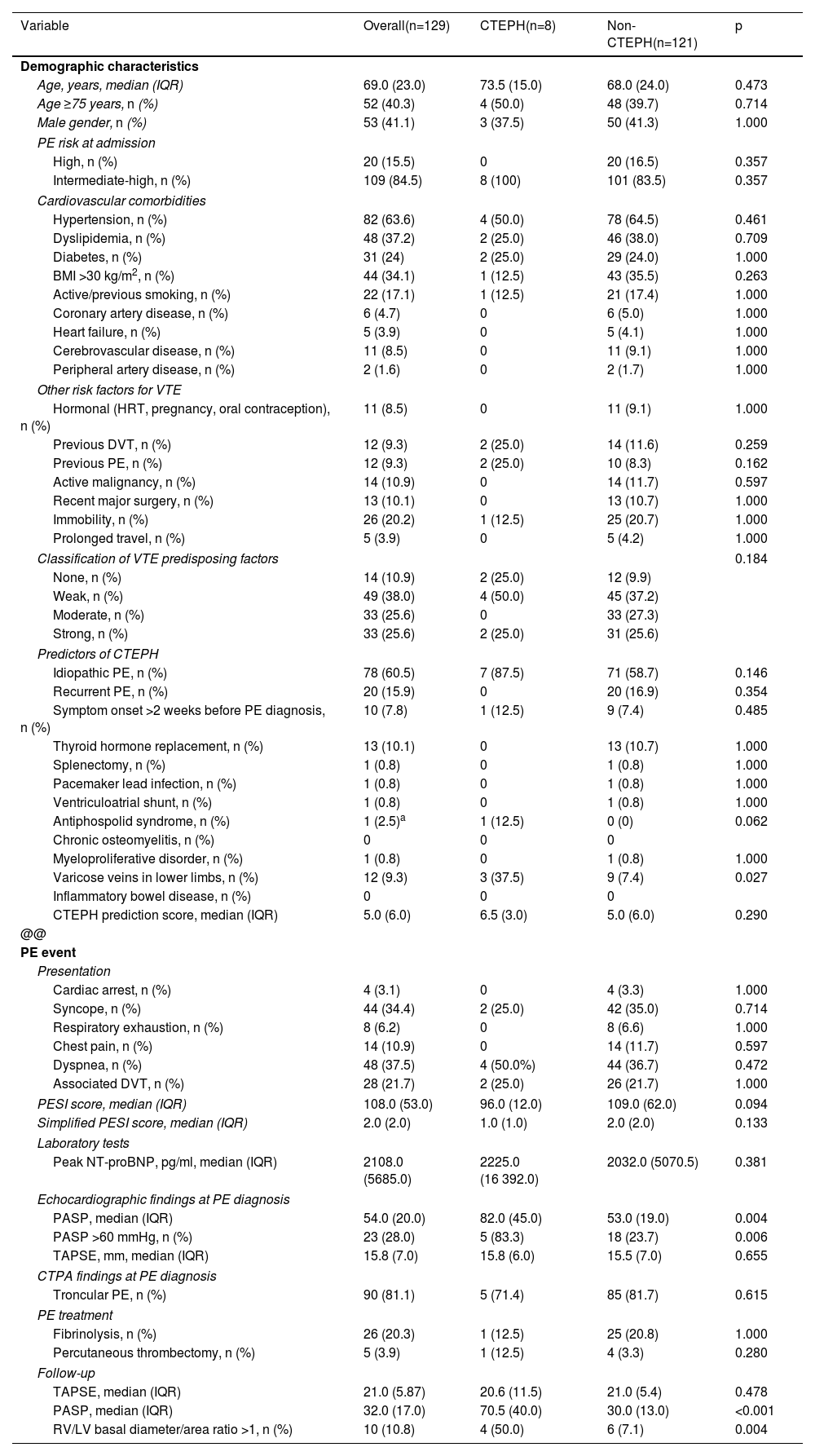
The baseline characteristics of the study population are summarized in Table 1.
Comparison of demographics and predictors of chronic thromboembolic pulmonary hypertension in the study population.
| Variable | Overall(n=129) | CTEPH(n=8) | Non-CTEPH(n=121) | p |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years, median (IQR) | 69.0 (23.0) | 73.5 (15.0) | 68.0 (24.0) | 0.473 |
| Age ≥75 years, n (%) | 52 (40.3) | 4 (50.0) | 48 (39.7) | 0.714 |
| Male gender, n (%) | 53 (41.1) | 3 (37.5) | 50 (41.3) | 1.000 |
| PE risk at admission | ||||
| High, n (%) | 20 (15.5) | 0 | 20 (16.5) | 0.357 |
| Intermediate-high, n (%) | 109 (84.5) | 8 (100) | 101 (83.5) | 0.357 |
| Cardiovascular comorbidities | ||||
| Hypertension, n (%) | 82 (63.6) | 4 (50.0) | 78 (64.5) | 0.461 |
| Dyslipidemia, n (%) | 48 (37.2) | 2 (25.0) | 46 (38.0) | 0.709 |
| Diabetes, n (%) | 31 (24) | 2 (25.0) | 29 (24.0) | 1.000 |
| BMI >30 kg/m2, n (%) | 44 (34.1) | 1 (12.5) | 43 (35.5) | 0.263 |
| Active/previous smoking, n (%) | 22 (17.1) | 1 (12.5) | 21 (17.4) | 1.000 |
| Coronary artery disease, n (%) | 6 (4.7) | 0 | 6 (5.0) | 1.000 |
| Heart failure, n (%) | 5 (3.9) | 0 | 5 (4.1) | 1.000 |
| Cerebrovascular disease, n (%) | 11 (8.5) | 0 | 11 (9.1) | 1.000 |
| Peripheral artery disease, n (%) | 2 (1.6) | 0 | 2 (1.7) | 1.000 |
| Other risk factors for VTE | ||||
| Hormonal (HRT, pregnancy, oral contraception), n (%) | 11 (8.5) | 0 | 11 (9.1) | 1.000 |
| Previous DVT, n (%) | 12 (9.3) | 2 (25.0) | 14 (11.6) | 0.259 |
| Previous PE, n (%) | 12 (9.3) | 2 (25.0) | 10 (8.3) | 0.162 |
| Active malignancy, n (%) | 14 (10.9) | 0 | 14 (11.7) | 0.597 |
| Recent major surgery, n (%) | 13 (10.1) | 0 | 13 (10.7) | 1.000 |
| Immobility, n (%) | 26 (20.2) | 1 (12.5) | 25 (20.7) | 1.000 |
| Prolonged travel, n (%) | 5 (3.9) | 0 | 5 (4.2) | 1.000 |
| Classification of VTE predisposing factors | 0.184 | |||
| None, n (%) | 14 (10.9) | 2 (25.0) | 12 (9.9) | |
| Weak, n (%) | 49 (38.0) | 4 (50.0) | 45 (37.2) | |
| Moderate, n (%) | 33 (25.6) | 0 | 33 (27.3) | |
| Strong, n (%) | 33 (25.6) | 2 (25.0) | 31 (25.6) | |
| Predictors of CTEPH | ||||
| Idiopathic PE, n (%) | 78 (60.5) | 7 (87.5) | 71 (58.7) | 0.146 |
| Recurrent PE, n (%) | 20 (15.9) | 0 | 20 (16.9) | 0.354 |
| Symptom onset >2 weeks before PE diagnosis, n (%) | 10 (7.8) | 1 (12.5) | 9 (7.4) | 0.485 |
| Thyroid hormone replacement, n (%) | 13 (10.1) | 0 | 13 (10.7) | 1.000 |
| Splenectomy, n (%) | 1 (0.8) | 0 | 1 (0.8) | 1.000 |
| Pacemaker lead infection, n (%) | 1 (0.8) | 0 | 1 (0.8) | 1.000 |
| Ventriculoatrial shunt, n (%) | 1 (0.8) | 0 | 1 (0.8) | 1.000 |
| Antiphospolid syndrome, n (%) | 1 (2.5)a | 1 (12.5) | 0 (0) | 0.062 |
| Chronic osteomyelitis, n (%) | 0 | 0 | 0 | |
| Myeloproliferative disorder, n (%) | 1 (0.8) | 0 | 1 (0.8) | 1.000 |
| Varicose veins in lower limbs, n (%) | 12 (9.3) | 3 (37.5) | 9 (7.4) | 0.027 |
| Inflammatory bowel disease, n (%) | 0 | 0 | 0 | |
| CTEPH prediction score, median (IQR) | 5.0 (6.0) | 6.5 (3.0) | 5.0 (6.0) | 0.290 |
| @@ | ||||
| PE event | ||||
| Presentation | ||||
| Cardiac arrest, n (%) | 4 (3.1) | 0 | 4 (3.3) | 1.000 |
| Syncope, n (%) | 44 (34.4) | 2 (25.0) | 42 (35.0) | 0.714 |
| Respiratory exhaustion, n (%) | 8 (6.2) | 0 | 8 (6.6) | 1.000 |
| Chest pain, n (%) | 14 (10.9) | 0 | 14 (11.7) | 0.597 |
| Dyspnea, n (%) | 48 (37.5) | 4 (50.0%) | 44 (36.7) | 0.472 |
| Associated DVT, n (%) | 28 (21.7) | 2 (25.0) | 26 (21.7) | 1.000 |
| PESI score, median (IQR) | 108.0 (53.0) | 96.0 (12.0) | 109.0 (62.0) | 0.094 |
| Simplified PESI score, median (IQR) | 2.0 (2.0) | 1.0 (1.0) | 2.0 (2.0) | 0.133 |
| Laboratory tests | ||||
| Peak NT-proBNP, pg/ml, median (IQR) | 2108.0 (5685.0) | 2225.0 (16 392.0) | 2032.0 (5070.5) | 0.381 |
| Echocardiographic findings at PE diagnosis | ||||
| PASP, median (IQR) | 54.0 (20.0) | 82.0 (45.0) | 53.0 (19.0) | 0.004 |
| PASP >60 mmHg, n (%) | 23 (28.0) | 5 (83.3) | 18 (23.7) | 0.006 |
| TAPSE, mm, median (IQR) | 15.8 (7.0) | 15.8 (6.0) | 15.5 (7.0) | 0.655 |
| CTPA findings at PE diagnosis | ||||
| Troncular PE, n (%) | 90 (81.1) | 5 (71.4) | 85 (81.7) | 0.615 |
| PE treatment | ||||
| Fibrinolysis, n (%) | 26 (20.3) | 1 (12.5) | 25 (20.8) | 1.000 |
| Percutaneous thrombectomy, n (%) | 5 (3.9) | 1 (12.5) | 4 (3.3) | 0.280 |
| Follow-up | ||||
| TAPSE, median (IQR) | 21.0 (5.87) | 20.6 (11.5) | 21.0 (5.4) | 0.478 |
| PASP, median (IQR) | 32.0 (17.0) | 70.5 (40.0) | 30.0 (13.0) | <0.001 |
| RV/LV basal diameter/area ratio >1, n (%) | 10 (10.8) | 4 (50.0) | 6 (7.1) | 0.004 |
BMI: body mass index; CTEPH: chronic thromboembolic pulmonary hypertension; CTPA: computed tomography pulmonary angiography; DVT: deep vein thrombosis; HRT: hormone replacement therapy; LV: left ventricular; NT-proBNP: N-terminal pro-brain natriuretic peptide; PASP: pulmonary artery systolic pressure; PE: pulmonary embolism; PESI: pulmonary embolism severity index; RV: right ventricular; TAPSE: tricuspid annular plane systolic excursion; VTE: venous thromboembolism.
During follow-up of the 129 patients diagnosed with intermediate-high or high-risk acute PE, four patients were diagnosed with CTEPH confirmed by RHC. Thus, a prevalence of 3.1% (n=4) of confirmed CTEPH following an intermediate-high or high-risk PE was found in our population. Additionally, four patients with compatible clinical presentation were found to have intermediate- or high-probability pulmonary hypertension on echocardiography and persistent mismatched perfusion defects on V/Q scan compatible with a diagnosis of CTEPH, but RHC was not performed for the following reasons: malignancy with poor prognosis, comorbidities with poor prognosis, advanced dementia or frailty. Therefore, a prevalence of suspected CTEPH of 6.2% (n=8) was estimated in our population following an intermediate-high or high-risk PE. The median time until diagnosis of CTEPH was 9.5 (6.0–21.0) months. Follow-up was completed at two years in 89.9% of patients.
Hemodynamic and clinical characteristics of patients with suspected CTEPH are summarized in Table 2.
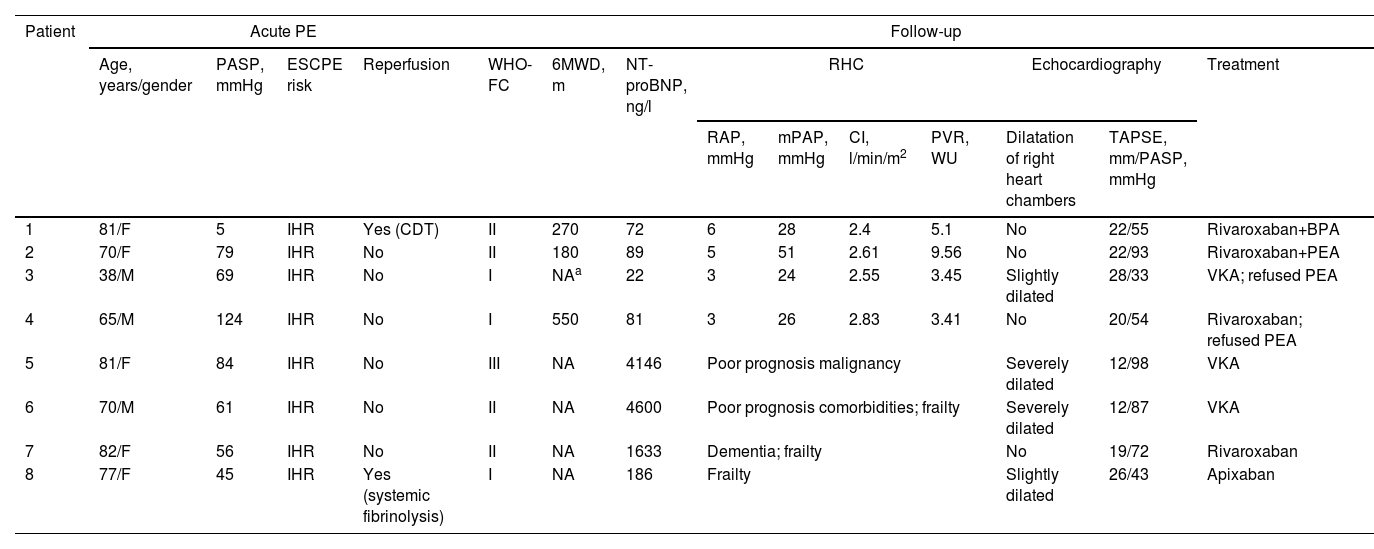
Individual characteristics of patients with chronic thromboembolic pulmonary hypertension.
| Patient | Acute PE | Follow-up | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years/gender | PASP, mmHg | ESCPE risk | Reperfusion | WHO-FC | 6MWD, m | NT-proBNP, ng/l | RHC | Echocardiography | Treatment | |||||
| RAP, mmHg | mPAP, mmHg | CI, l/min/m2 | PVR, WU | Dilatation of right heart chambers | TAPSE, mm/PASP, mmHg | |||||||||
| 1 | 81/F | 5 | IHR | Yes (CDT) | II | 270 | 72 | 6 | 28 | 2.4 | 5.1 | No | 22/55 | Rivaroxaban+BPA |
| 2 | 70/F | 79 | IHR | No | II | 180 | 89 | 5 | 51 | 2.61 | 9.56 | No | 22/93 | Rivaroxaban+PEA |
| 3 | 38/M | 69 | IHR | No | I | NAa | 22 | 3 | 24 | 2.55 | 3.45 | Slightly dilated | 28/33 | VKA; refused PEA |
| 4 | 65/M | 124 | IHR | No | I | 550 | 81 | 3 | 26 | 2.83 | 3.41 | No | 20/54 | Rivaroxaban; refused PEA |
| 5 | 81/F | 84 | IHR | No | III | NA | 4146 | Poor prognosis malignancy | Severely dilated | 12/98 | VKA | |||
| 6 | 70/M | 61 | IHR | No | II | NA | 4600 | Poor prognosis comorbidities; frailty | Severely dilated | 12/87 | VKA | |||
| 7 | 82/F | 56 | IHR | No | II | NA | 1633 | Dementia; frailty | No | 19/72 | Rivaroxaban | |||
| 8 | 77/F | 45 | IHR | Yes (systemic fibrinolysis) | I | NA | 186 | Frailty | Slightly dilated | 26/43 | Apixaban | |||
6MWD: six-minute walk test; BPA: balloon pulmonary angioplasty; CDT: catheter-directed thrombolysis; CI: cardiac index; CTEPH: chronic thromboembolic pulmonary hypertension; ESC: European Society of Cardiology; F: female; HR: high risk; IHR: intermediate-high risk; M: male; mPAP: mean pulmonary artery pressure; NA: not available; NT-proBNP: N-terminal pro-brain natriuretic peptide; PASP: pulmonary artery systolic pressure; PE: pulmonary embolism; PEA: pulmonary thromboendarterectomy; PVR: pulmonary vascular resistance; RAP: right arterial pressure; RHC: right heart catheterization; TAPSE: tricuspid annular plane systolic excursion; VKA: vitamin K antagonist; WHO-FC: World Health Organization functional class; WU: Wood units.
Among patients with confirmed CTEPH, all were anticoagulated (three on direct oral anticoagulants and one on a vitamin K antagonist). One underwent pulmonary thromboendarterectomy (PEA) and another underwent balloon pulmonary angioplasty, both with significant clinical and hemodynamic improvement after the procedures. The other two patients refused PEA. No patient is currently medicated with pulmonary vasodilators.
Analysis of risk factorsThe comparison between patients with and without suspected CTEPH is outlined in Table 1.
Older age was found in patients with CTEPH, with a median age of 73.5 years (IQR 15.0) in CTEPH patients compared to 68.0 years (IQR 24.0) in patients without CTEPH. Patients with CTEPH were all admitted with intermediate-high risk PE, whereas 16.5% of patients not diagnosed with CTEPH were stratified as having high-risk PE (p=0.357).
Analysis of CTEPH predictors revealed some significant differences. A higher incidence of varicose veins in the lower limbs was described in patients who developed CTEPH (37.5%) than in those not diagnosed with CTEPH (p=0.027). Idiopathic PE was more frequently associated with CTEPH, although not significantly (p=0.146). Antiphospholipid syndrome was diagnosed in one patient (12.5%) with CTEPH, while no patients without CTEPH had this comorbidity (p=0.062). Median pulmonary artery systolic pressure (PASP) in patients diagnosed with CTEPH was 82.0 mmHg (IQR 45.0), compared to 53.0 mmHg (IQR 19.0) in patients without CTEPH (p=0.004). Increased PASP at admission (OR 1.12; 95% CI 1.04–1.22; p=0.005) and the presence of varicose veins in the lower limbs (OR 7.47; 95% CI 1.53–36.41; p=0.013) were predictors of CTEPH in multivariate analysis.
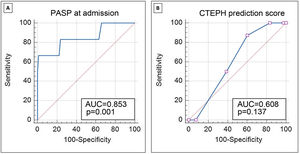
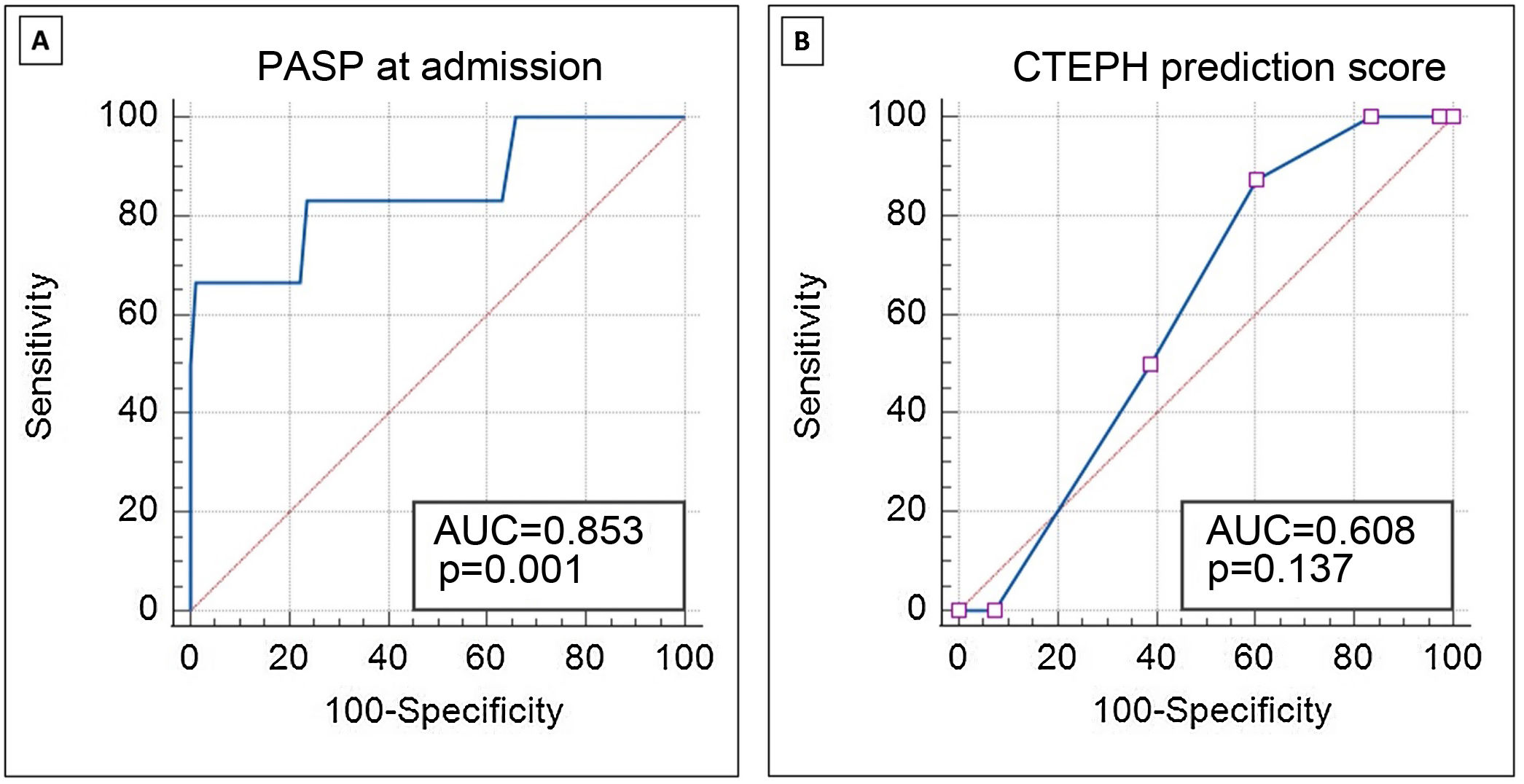
ROC curve analysis (Figure 2A) demonstrates the good discriminative ability of PASP at acute PE admission to predict CTEPH during follow-up (area under the ROC curve 0.85; 95% CI 0.76–0.92; p=0.001). PASP >60 mmHg at admission identified patients with CTEPH during follow-up with sensitivity and specificity of 83.3% and 76.3%, respectively. PASP >79 mmHg at the index event identified CTEPH with sensitivity of 66.7% and specificity of 98.7%.
Receiver operating characteristic curve analysis demonstrating the discriminative ability of PASP at admission for acute PE (A) and the CTEPH prediction score (B) to predict the presence of CTEPH in follow-up. AUC: area under the curve; CTEPH: chronic thromboembolic pulmonary hypertension; PASP: pulmonary artery systolic pressure.
The CTEPH prediction score in CTEPH patients was a median 6.5 compared to 5.0 in patients not diagnosed with CTEPH (p=0.290). This score (Figure 2B) did not show a good discriminative ability in our population to predict CTEPH in the follow-up (area under the ROC curve 0.61; 95% CI 0.52–0.69; p=0.137).
No significant difference was found between fibrinolysis and percutaneous thrombectomy in the two groups.
At follow-up, higher PASP levels were found in patients with CTEPH, with a median of 70.5 mmHg (IQR 40.0), compared to a median of 30.0 mmHg (IQR 13.0) in patients with no CTEPH (p<0.001). Additionally, a right ventricular/left ventricular basal diameter/area ratio >1 in the follow-up was also statistically significant in predicting CTEPH development. Four of the patients with CTEPH (50%) had dilated right heart chambers compared to six (7.1%) of those not diagnosed with CTEPH (p=0.004).
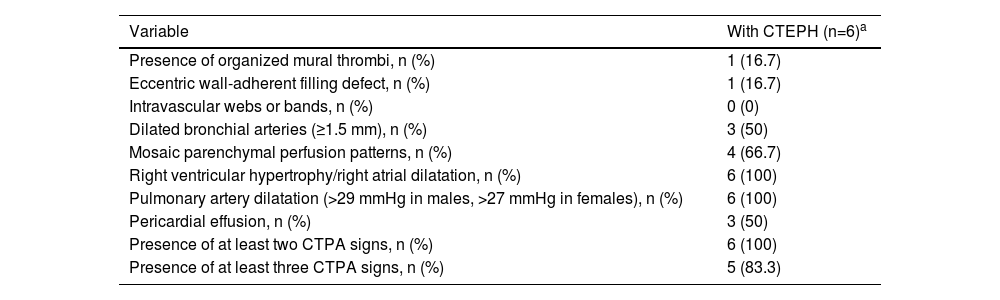
Computed tomography pulmonary angiography findings at the time of index pulmonary embolism in patients with chronic thromboembolic pulmonary hypertensionMultidetector CTPA findings from the index PE in patients with suspected CTEPH were analyzed by two radiologists (Table 3). In all patients at least two signs of CTEPH were detected on the multidetector CTPA and in five of them there were at least three findings suggestive of CTEPH.
Signs of chronic thromboembolic pulmonary hypertension on computed tomography pulmonary angiography at the time of the pulmonary embolism event.
| Variable | With CTEPH (n=6)a |
|---|---|
| Presence of organized mural thrombi, n (%) | 1 (16.7) |
| Eccentric wall-adherent filling defect, n (%) | 1 (16.7) |
| Intravascular webs or bands, n (%) | 0 (0) |
| Dilated bronchial arteries (≥1.5 mm), n (%) | 3 (50) |
| Mosaic parenchymal perfusion patterns, n (%) | 4 (66.7) |
| Right ventricular hypertrophy/right atrial dilatation, n (%) | 6 (100) |
| Pulmonary artery dilatation (>29 mmHg in males, >27 mmHg in females), n (%) | 6 (100) |
| Pericardial effusion, n (%) | 3 (50) |
| Presence of at least two CTPA signs, n (%) | 6 (100) |
| Presence of at least three CTPA signs, n (%) | 5 (83.3) |
CTEPH: chronic thromboembolic pulmonary hypertension; CTPA: computed tomography pulmonary angiography.
To our knowledge, this is the first analysis to report the prevalence of PE according to risk stratification in a Portuguese population, which is similar to that reported in international studies.21
The main finding of this retrospective cohort study of patients hospitalized due to intermediate-high or high-risk PE in a national referral center for pulmonary hypertension between 2014 and 2019 is an overall prevalence of 6.2% of suspected CTEPH by clinical assessment, Doppler echocardiography and V/Q lung scan, and confirmed CTEPH of 3.1% by RHC, among patients who were followed for a median of 41 months after the index PE.
According to recent studies, the cumulative incidence of CTEPH following symptomatic PE is 0.1–9.1% two years after the acute event,3,7,9,22 estimates that differ substantially between studies and geographic regions.16,22 Higher prevalences are found in studies with no RHC confirmation and based solely on echocardiographic findings, with PASP cut-off values having a significant impact on CTEPH incidence.12,14 The prevalence estimated in our study is therefore consistent with other studies that confirmed CTEPH by RHC and is lower than those in which RHC was not performed. Some published studies suffer from selection bias, including the exclusion of patients with possible causes of other forms of pulmonary hypertension or pre-existing exertional dyspnea5,17,23 and with permanent risk factors for venous thromboembolism (VTE), including cancer or thrombophilia.24 Our study did not exclude patients with increased risk of VTE recurrence or those with other possible causes of pulmonary hypertension.
The only study conducted in a Portuguese population, by Barros et al., which reported an incidence of 12.4% of pulmonary hypertension following an intermediate- or high-risk PE, has recognizable limitations, including the absence of confirmation by RHC and the inclusion only of patients admitted to the coronary intensive care unit.25 In our study we included consecutive patients hospitalized with PE, regardless of the unit or department in which they were cared for. Furthermore, we were able to confirm the diagnosis of CTEPH by RHC in four of the eight patients with suspected CTEPH.
Diagnosis of CTEPH is hampered in the early stages by the nonspecificity of symptoms, as symptoms of right heart failure only present in advanced stages.3,14 Delay in CTEPH diagnosis is associated with increased morbidity and mortality.16,26 Therefore, timely recognition and management are of utmost importance. Time to diagnosis in our study was a median of 9.5 months, which is in line with the current evidence.27
The differences in documented incidences of CTEPH preclude the establishment of appropriate standardized screening protocols for CTEPH after a PE event. Universal implementation of long-term echocardiographic follow-up in all PE survivors has been shown to be cost-ineffective and has a low diagnostic yield, hence, echocardiography for all patients with a history of PE is not warranted.13,18 The CTEPH prediction score was accordingly developed to exclude CTEPH without the need for echocardiography.18 Unfortunately, in our study we found that the prediction score did not show a good discriminatory ability to predict CTEPH following a more severe form of PE. We attribute this to the fact that our population only included the most severe forms of PE, in contrast to the inShape study II, in which only 2.2% of patients had high-risk PE. Furthermore, the score includes right ventricular dysfunction, a characteristic present in all our patients and therefore not discriminatory in our population. Hence, our study suggests that further prediction scores are required to predict the risk of CTEPH following a more severe PE event.
Several potential predisposing factors for CTEPH following a PE event have been reported in the literature, although with substantial discrepancies between studies. Our study identified some of the predictors of CTEPH, supporting the need for a more cautious follow-up when identified.
Age remains a controversial risk factor for the development of CTEPH in the literature. In our study we found no statistically significant differences in age between patients who developed CTEPH and those who did not. However, we found that patients with CTEPH showed a tendency to be older, as suggested in numerous studies.17,25,28,29
In our population, all of those who developed CTEPH had intermediate-high risk PE. None of the patients presenting with high-risk PE developed CTEPH. As observed by Yang et al.,30 thrombolysis applied to high-risk patients may explain this finding, as it increases thrombus dissolution in comparison to anticoagulation alone. In our study, most patients who developed CTEPH did not receive thrombolysis and were only anticoagulated. In our analysis it was not possible to identify thrombolysis performed at the time of the acute PE as a protective factor for the development of CTEPH, probably because the small number of patients who developed CTEPH hindered identification of independent predictors for the disease. To date, the role of thrombolysis remains unclear regarding the risk of developing CTEPH.13,30–32 The MOPPETT study,33 Klok et al.10 and Sharma et al.34 reported a lower incidence of CTEPH following an intermediate-risk PE treated by thrombolysis, in contrast to more recent studies.35 Further studies are required to assess the impact of thrombolysis on CTEPH development following a PE event and whether performing thrombolysis in intermediate-high risk patients would protect them from developing CTEPH.
Although the rate of idiopathic PE in our study did not differ statistically between the two groups, we noted that seven out of eight patients with CTEPH had an idiopathic PE. As proposed in other studies,22 idiopathic PE increases the risk of CTEPH, as the underlying disease is not treated, resulting in continuous thrombus formation.
Varicose veins in the lower limbs were found to predict the development of CTEPH in our population, similarly to other studies.28,30 Blood stasis created by the varicose veins may produce emboli that gradually migrate to the pulmonary vasculature, resulting in recurring undiagnosed PE events. As reported by some authors,36 clinically silent PE is associated with greater risk of CTEPH because of undertreatment.
Furthermore, although not statistically significant, the presence of antiphospholipid syndrome was associated with increased risk of CTEPH development, supporting the results of other studies with the same conclusion.37
Patients with higher PASP at diagnosis showed increased risk of developing CTEPH. The presence of PASP >60 mmHg is unusual in the setting of an acute PE event because the normal right ventricle is unable to maintain a PASP >40 mmHg, even when there is massive PE with angiographically estimated vascular obstruction over 50%.5,38 Hence, higher PASP at admission can increase the specificity for detecting CTEPH in follow-up, as demonstrated in our analyses (cut-off >79 mmHg showed a specificity of 99%). As proposed by some studies17,39 and the most recent guidelines,2 such high pressures reflect chronic obstruction of the pulmonary vasculature and support the hypothesis of pre-existing CTEPH at the time of acute PE.
Most patients who develop CTEPH after PE have been found to have various signs of CTEPH at the initial CTPA in several studies,17,19 which suggests a primary diagnosis of an acute-on-chronic event rather than acute PE.4 As proposed in the literature, identification of ≥3 radiological characteristics of CTEPH on the initial CTPA at the time of the suspected PE event virtually diagnoses this entity (specificity 96%).19 Therefore, analysis of appropriate CTPA protocols at the initial event is crucial for its early recognition and proper management, as suggested in the current guidelines.2 In our study we found that all patients had at least two signs of CTEPH at the time of the index event, again suggesting the presence of CTEPH at the time of the index PE (acute-on-chronic PE). Although not compared to non-CTEPH patients, the radiological predictors of CTEPH described in other studies and recent guidelines19,20 were present in some of our patients, supporting their predictive ability.
In contrast to other studies,17 NT-proBNP at the PE event was not a predictor of CTEPH in our cohort. However, dilatation of the right chambers at follow-up was associated with increased risk of CTEPH, reflecting the adaptation of the right ventricle to high pressures in the pulmonary vasculature.
Study limitationsDespite the limitations inherent to a retrospective analysis, this is the first study to estimate the prevalence of CTEPH after a PE event confirmed by RHC in a Portuguese population. However, we recognize some limitations in our study that should be addressed.
The main limitation of our work was the small number of patients with CTEPH, which precluded the identification of predictors for this entity. Due to this small sample, multivariate analysis did not show statistical differences in multiple predictors that in other studies were associated with increased risk of developing CTEPH. Further studies are required for additional research into predictors of CTEPH following a more severe form of PE.
Secondly, it was not possible to confirm the diagnosis of CTEPH by RHC in all patients with suspected CTEPH, due to the comorbidities present in some patients that prevented them from undergoing this invasive procedure. For this reason, in individuals unable to undergo the invasive procedure, CTEPH was defined as the presence of echocardiographic findings suggestive of pulmonary hypertension (including peak tricuspid regurgitation velocity >2.9 m/s) and positive findings on the V/Q scan. Even though this alternate diagnosis is not the gold standard for diagnosis and therefore not as accurate as RHC, the exclusion of patients who were unable to undergo RHC due to comorbidities would greatly underestimate the prevalence of CTEPH following acute PE.
Thirdly, only CTEPH patients had their CTPA analyzed by expert radiologists, which precludes multivariate analysis of radiological predictors of CTEPH, which have been undertaken in other studies.
ConclusionsIn our cohort, the prevalence of CTEPH in survivors of severe forms of acute PE was 6.2%. The presence of varicose veins and PASP >60 mmHg at admission for the index event were identified as early predictors of CTEPH. Our study therefore suggests that patients with these features should be followed carefully to exclude CTEPH and physicians should be alert for the development of symptoms that may be attributed to CTEPH. Further studies are needed to confirm the validity of these and other predisposing factors that could be addressed to prevent the occurrence of CTEPH. Furthermore, radiological findings of CTEPH may be present at the index event, suggesting a diagnosis of acute-on-chronic PE and further assisting in the earlier diagnosis and timely management of CTEPH. Additionally, the CTEPH prediction score did not show a good discriminatory ability to predict CTEPH in our study, suggesting that other prediction scores are required to predict the risk of CTEPH following a more severe PE event.
FundingThe study has no funding to declare.
Conflicts of interestThe authors have no conflicts of interest to declare.




![Study flowchart. * Risk stratification at admission following the current ESC guidelines, although PESI score was not considered for this categorization: low risk (absence of right ventricular [RV] dysfunction and troponin elevation); intermediate-low risk (RV dysfunction on transthoracic echocardiogram [TTE] or computed tomography pulmonary angiography [CTPA]; or elevated troponin levels); intermediate-high risk PE (RV dysfunction on TTE or CTPA and elevated troponin levels); high-risk (cardiac arrest: need for cardiopulmonary resuscitation; obstructive shock: hypotension (systolic blood pressure [BP] <90 mmHg) or vasopressors required to achieve BP >90 mmHg despite adequate filling status) and end-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria; increased serum lactate); persistent hypotension (systolic BP <90 mmHg or systolic BP drop >40 mmHg, lasting longer than 15 min and not caused by new-onset arrhythmia, hypovolemia, or sepsis). CTEPH: chronic thromboembolic pulmonary hypertension; PE: pulmonary embolism; RHC: right heart catheterization. Study flowchart. * Risk stratification at admission following the current ESC guidelines, although PESI score was not considered for this categorization: low risk (absence of right ventricular [RV] dysfunction and troponin elevation); intermediate-low risk (RV dysfunction on transthoracic echocardiogram [TTE] or computed tomography pulmonary angiography [CTPA]; or elevated troponin levels); intermediate-high risk PE (RV dysfunction on TTE or CTPA and elevated troponin levels); high-risk (cardiac arrest: need for cardiopulmonary resuscitation; obstructive shock: hypotension (systolic blood pressure [BP] <90 mmHg) or vasopressors required to achieve BP >90 mmHg despite adequate filling status) and end-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria; increased serum lactate); persistent hypotension (systolic BP <90 mmHg or systolic BP drop >40 mmHg, lasting longer than 15 min and not caused by new-onset arrhythmia, hypovolemia, or sepsis). CTEPH: chronic thromboembolic pulmonary hypertension; PE: pulmonary embolism; RHC: right heart catheterization.](https://static.elsevier.es/multimedia/08702551/0000004200000012/v3_202312120610/S087025512300392X/v3_202312120610/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)