During the COVID-19 pandemic, among the safety measures adopted, use of facemasks during exercise training sessions in cardiac rehabilitation programs raised concerns regarding possible detrimental effects on exercise capacity. Our study examined the cardiorespiratory impact of wearing two types of the most common facemasks during treadmill aerobic training.
MethodsTwelve healthy health professionals completed three trials of a symptom-limited Bruce treadmill protocol: Without a mask, with a surgical mask and with a respirator. Perceived exertion and dyspnea were evaluated with the Borg Scale of Perceived Exertion and the Borg Dyspnea Scale, respectively. Blood pressure, heart rate and arterial oxygen saturation (SpO2) were measured at each 3-minute stage.
ResultsUsing a surgical mask or a respirator resulted in a shorter duration of exercise testing. At peak capacity, using a respirator resulted in higher levels of dyspnea and perceived exertion compared to not wearing a facemask. A significant drop in SpO2 was present at the end of exercise testing only when using a respirator. There were no differences in either chronotropic or blood pressure responses between testing conditions.
ConclusionsProfessionals involved in cardiac rehabilitation should be aware of the cardiorespiratory impact of facemasks. Future studies should assess whether exposure to these conditions may impact on the overall results of contemporary cardiac rehabilitation programs.
Durante a pandemia Covid-19 a utilização de máscaras faciais, incluindo durante o exercício terapêutico, faz parte das medidas de segurança adotadas. Este facto originou preocupação a nível das unidades de reabilitação cardíaca, uma vez que as máscaras faciais podem promover efeitos deletérios na capacidade de exercício. Este estudo avaliou o impacto da utilização das máscaras faciais durante o treino aeróbio em passadeira.
MétodosDoze profissionais de saúde saudáveis completaram três provas em passadeira de acordo com o protocolo de Bruce: sem máscara, com máscara cirúrgica e com um respirador. A perceção de esforço e dispneia foi avaliada com a Escala de Perceção de Esforço de Borg e com a Escala de Dispneia de Borg, respetivamente. A pressão arterial, frequência cardíaca e saturação arterial de oxigénio (SpO2) foram registadas em cada estadio do protocolo.
ResultadosA utilização de máscara facial resultou numa menor duração da prova e, em determinados momentos, níveis de perceção de dispneia e de esforço mais elevados. Verificou-se uma descida significativa da SpO2 no final da prova com respirador. Não se verificaram diferenças na resposta cronotrópica ou da pressão arterial entre as diferentes condições de prova.
ConclusõesOs profissionais envolvidos na reabilitação cardíaca devem reconhecer os impactos cardiorrespiratórios provocados pela utilização da máscara facial. São necessários mais estudos para determinar se a exposição a estas condições de treino pode ter impacto nos resultados dos programas de reabilitação cardíaca.
Cardiac rehabilitation programs (CRP) are widely recommended as part of the optimal treatment of patients with cardiovascular (CV) disease, particularly after an acute coronary syndrome.1 These patients often have a lower exercise tolerance and require a higher exertion to perform the same level of activity compared to a healthy individual,2 and these limitations can be effectively overturned by participation in exercise-based CRP. The COVID-19 (SARS-CoV-2 associated disease) pandemic has forced the suspension of most, if not all, group exercise training sessions due to concerns over spreading SARS-CoV-2.3,4 Nevertheless, resumption of CRP is warranted, provided safety measures are adopted, including the use of facemasks (FM) during exercise training sessions.3,5 This has been increasingly recognized and recommended as a mitigation strategy to overcome the potential aerosolization associated with the higher expiratory flows generated during submaximal and maximal exercise.6,7 However, the ability to exercise in these conditions raises some concerns. Rebreathing of low-oxygen high-carbon dioxide expired air may interfere with alveolar gas diffusion and blood oxygen uptake.8 On the other hand, increased resistance to air flow resulting from using FM can lead to increased respiratory effort and early respiratory muscle fatigue.8 This combination could potentially result in an impaired exercise performance, although there are mixed results on this topic.9 Our study aimed to assess the impact of using different FMs (surgical mask (SM) and respirator (R)), compared to not using a FM, on the cardiorespiratory physiological response and rate of perceived exertion and dyspnea during treadmill aerobic training in healthy subjects.
Materials and methodsSubjectsWe recruited a consecutive sample of healthy health professionals from our department. A brief review of clinical data was obtained using a structured questionnaire regarding past medical history and medication. Physical activity was assessed by the Portuguese version of the International Physical Activity Questionnaire (IPAQ), which classifies leisure time physical activity levels as low, moderate or high.10,11 None of the subjects had performed an exercise test on treadmill in the previous twelve months. Sample size calculation with the power to detect differences between groups of 80% with a level of significance of 0.05, was calculated assuming a mean difference in overall exercise discomfort score between not wearing a FM and using either SM or R of 2.4 and a standard deviation (SD) of 2.0,12 resulting in at least 12 observations for each time point.
Surgical masks and respiratorsOur study aimed to compare the physiological response and perceived exertion in an incremental exercise protocol in three testing conditions: Not wearing a FM (group 1: without mask [WM]) versus using two different types of FM with different filtration properties. A disposable three-layer type 2 SM (MASK-98 model, manufactured by Razi Protect, in Portugal) (group 2: SM), with a minimum filter capacity of 95% of particles 3.0 microns or larger in diameter, and a R with a filtering facepiece score of 2 (FFP2) (group 3: R) were used. The R available at the time of the study as the Chinese KN95 (GB2626-2006 model, manufactured by Lianyungang Manai Protective Equipment Company, in China), which filtrates a minimum of 94% of all particles >0.3 microns or larger in diameter – an equivalent safety specification to the European FFP2 respirators.13 The KN95 respirators were therefore considered adequate for this study.
Study protocolEach subject performed three tests: One in regular conditions, without a FM; one with a SM; and another one with a KN95 respirator. Each test was performed with at least a 48 hour interval and preferably at the same time of day. The subjects were asked not to perform vigorous exercise in the 24 hours prior to each test. The sequence of tests was randomized to reduce bias, using a computerized random number generator, with allocation known to the examiner and patient only at the time of the test.
For exercise testing (ET) we followed the Bruce treadmill protocol (using the Mill & Mill® Track model, manufactured by Lode B.V., in the Netherlands). Handrail support was allowed only for balance. At rest, at the last minute of each of the three-minute stages of the Bruce protocol and at peak exercise, we recorded heart rate (HR), blood pressure (BP) and arterial oxygen saturation (SpO2) and quantified level of fatigue and dyspnea using the Borg Scale of Perceived Exertion and the Borg Dyspnea Scale, respectively. BP was measured with an upper-arm manual BP cuff (Big Ben Round model, manufactured by Riester, in Germany), in the left arm, and HR and SpO2 with a finger oximeter worn throughout the test (GIMA PC-68B). Subjects were allowed ET warm up period at 2.7 km/h with 0% grade for three minutes and performed a cool down period of 5 minutes at the end. BP, HR and SpO2 were recorded again during the cool down period (after three minutes for BP and after one and three minutes for HR and SpO2). The investigators followed the American Heart Association criteria for ET termination.14 All data was registered during each test in a blinded form (no identifiable information on the subject), coded using a single randomly generated combination of digits and characters and inserted into the database by a different member of the investigator team not otherwise involved in any step of the study.
All participants were informed of the procedures and potential risks before testing and a written informed consent was obtained as per institution protocol. All safety measures regarding ET were adopted.
Statistical analysisStandard descriptive measures, namely mean and standard deviation (SD) for normally distributed continuous variables and proportion (expressed as %) for categorical variables were used. Normality assessment was done using the Kolmogorov-Smirnov test and visual inspection of the distribution histogram. To account for the dependence of observations, since the same subject was tested in three study conditions, a repeated measure analysis of variance was used. For within-subjects’ differences in each timepoint F statistics were used considering a significance p<0.05. Furthermore, to allow for between-group comparisons (WM, SM, R), a Bonferroni adjustment analysis was performed.
Each patient exercise test was categorized according to a percentage of the total time achieved (25%, 50%, 75% and 100%), independently of its duration, and data was collected in each of these timepoints. This approach was chosen since not all subjects completed the total protocol time, leaving fewer observations in the last stages of the exercise test.
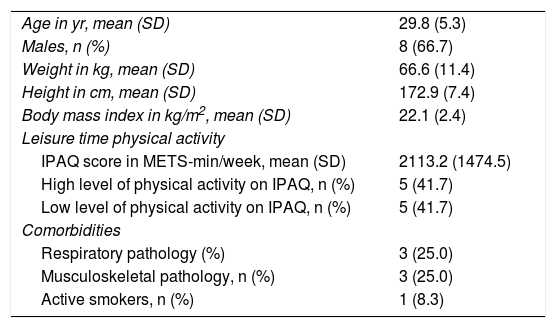
ResultsPopulation characteristicsTwelve subjects were recruited. Baseline characteristics are presented in Table 1. The study sample consisted of young (age range: 25-45 years), mostly male health professionals. All subjects had normal body mass index, ranging between 19.9 kg/m2 and 24.7 kg/m2, with the majority (seven subjects (58.3%)) being moderately to highly physically active. Two subjects had previous respiratory disorders, namely one subject with asthma (medicated with a leukotriene receptor antagonist – montelukast, 10 mg per day, with no recent exacerbations) and another one with allergic rhinitis. Concerning musculoskeletal diseases, one subject had a history of a healed peroneal fracture, one subject had a history of previous surgical treatment for a lumbar disk herniation and one had a recent history of paratendinopathy of the Achilles tendon. All subjects performed the three tests and there were no dropouts.
Baseline characteristics of study sample.
| Age in yr, mean (SD) | 29.8 (5.3) |
| Males, n (%) | 8 (66.7) |
| Weight in kg, mean (SD) | 66.6 (11.4) |
| Height in cm, mean (SD) | 172.9 (7.4) |
| Body mass index in kg/m2, mean (SD) | 22.1 (2.4) |
| Leisure time physical activity | |
| IPAQ score in METS-min/week, mean (SD) | 2113.2 (1474.5) |
| High level of physical activity on IPAQ, n (%) | 5 (41.7) |
| Low level of physical activity on IPAQ, n (%) | 5 (41.7) |
| Comorbidities | |
| Respiratory pathology (%) | 3 (25.0) |
| Musculoskeletal pathology, n (%) | 3 (25.0) |
| Active smokers, n (%) | 1 (8.3) |
Abbreviations: IPAQ: International Physical Activity Questionnaire; METS: metabolic equivalents.
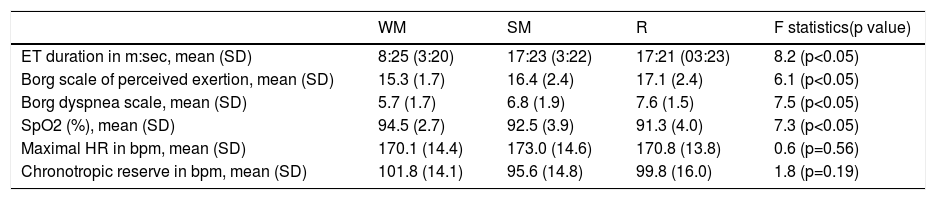
Our study found that, compared to not wearing a FM, wearing a FM was associated with shorter ET duration, independent of the type of mask worn (Table 2). There was no significant difference in ET duration between wearing SM or R. All patients completed the third stage of the Bruce protocol, with dropouts beginning at stage four, when using a FM, and at stage five when not wearing a mask. The Bruce protocol was completed by seven (50.3%) subjects when WM, but only by four (33.3%) when wearing a SM or a R.
Comparison of variables measured at the end of the exercise testing protocol between each of the test conditions.
| WM | SM | R | F statistics(p value) | |
|---|---|---|---|---|
| ET duration in m:sec, mean (SD) | 8:25 (3:20) | 17:23 (3:22) | 17:21 (03:23) | 8.2 (p<0.05) |
| Borg scale of perceived exertion, mean (SD) | 15.3 (1.7) | 16.4 (2.4) | 17.1 (2.4) | 6.1 (p<0.05) |
| Borg dyspnea scale, mean (SD) | 5.7 (1.7) | 6.8 (1.9) | 7.6 (1.5) | 7.5 (p<0.05) |
| SpO2 (%), mean (SD) | 94.5 (2.7) | 92.5 (3.9) | 91.3 (4.0) | 7.3 (p<0.05) |
| Maximal HR in bpm, mean (SD) | 170.1 (14.4) | 173.0 (14.6) | 170.8 (13.8) | 0.6 (p=0.56) |
| Chronotropic reserve in bpm, mean (SD) | 101.8 (14.1) | 95.6 (14.8) | 99.8 (16.0) | 1.8 (p=0.19) |
Abbreviations: bpm: beats per minute; ET: exercise testing; HR: heart rate; R: respirator; SM: surgical mask; SpO2: arterial oxygen saturation; WM: without mask.
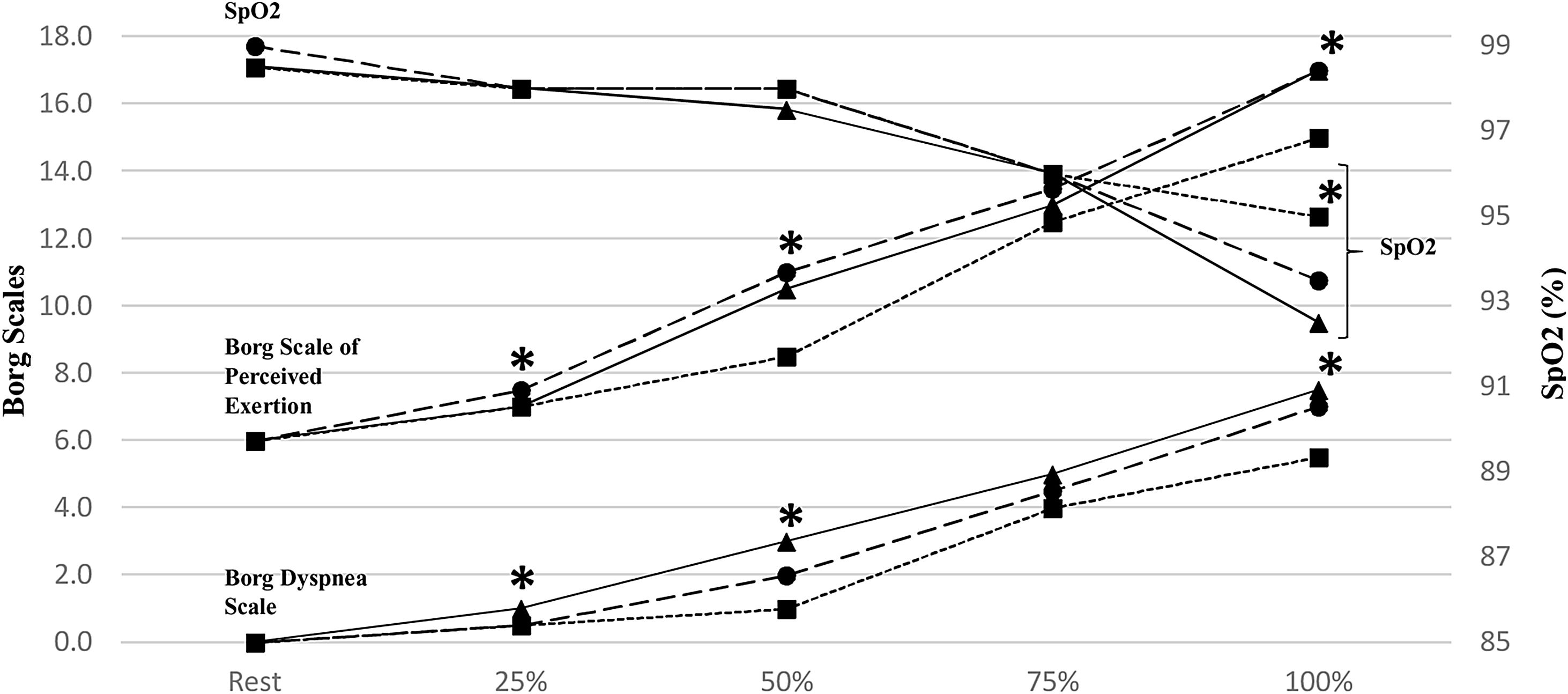
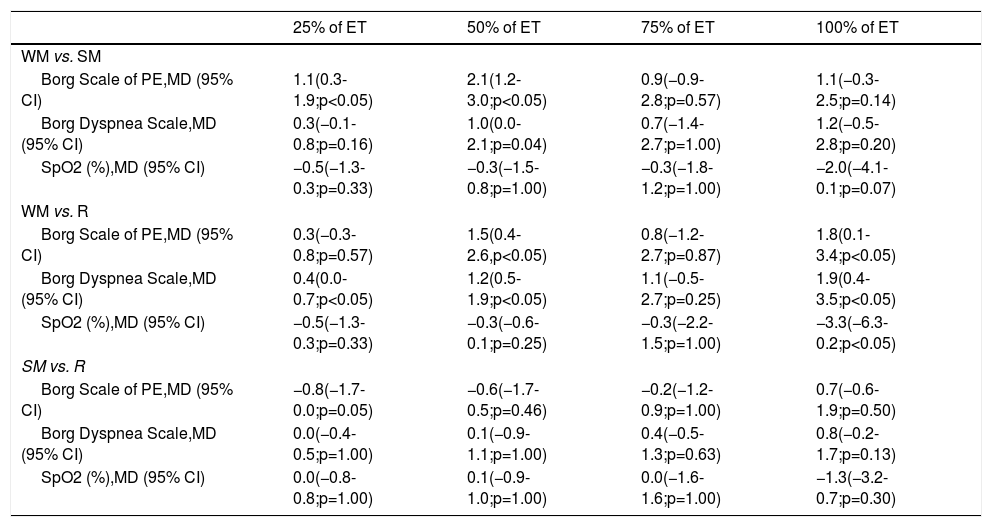
At 25% of total time achieved in ET, only wearing a SM showed a significant higher level of exercise perception compared to not wearing a FM (Table 3). At 50% of total time achieved in ET, both wearing a SM or a R showed a significantly higher level of perceived exertion compared to WM (Table 3). At 75% of test completion, no differences between testing conditions (p=0.14) were found. At the end of the ET, wearing a R was associated with a significantly higher level of perceived exertion when compared to WM (Table 3). No differences were found in any stage of ET between wearing SM and R.
Mean differences in Borg Scale scores and SpO2 for each test condition at point of the exercise test.
| 25% of ET | 50% of ET | 75% of ET | 100% of ET | |
|---|---|---|---|---|
| WM vs. SM | ||||
| Borg Scale of PE,MD (95% CI) | 1.1(0.3-1.9;p<0.05) | 2.1(1.2-3.0;p<0.05) | 0.9(−0.9-2.8;p=0.57) | 1.1(−0.3-2.5;p=0.14) |
| Borg Dyspnea Scale,MD (95% CI) | 0.3(−0.1-0.8;p=0.16) | 1.0(0.0-2.1;p=0.04) | 0.7(−1.4-2.7;p=1.00) | 1.2(−0.5-2.8;p=0.20) |
| SpO2 (%),MD (95% CI) | −0.5(−1.3-0.3;p=0.33) | −0.3(−1.5-0.8;p=1.00) | −0.3(−1.8-1.2;p=1.00) | −2.0(−4.1-0.1;p=0.07) |
| WM vs. R | ||||
| Borg Scale of PE,MD (95% CI) | 0.3(−0.3-0.8;p=0.57) | 1.5(0.4-2.6,p<0.05) | 0.8(−1.2-2.7;p=0.87) | 1.8(0.1-3.4;p<0.05) |
| Borg Dyspnea Scale,MD (95% CI) | 0.4(0.0-0.7;p<0.05) | 1.2(0.5-1.9;p<0.05) | 1.1(−0.5-2.7;p=0.25) | 1.9(0.4-3.5;p<0.05) |
| SpO2 (%),MD (95% CI) | −0.5(−1.3-0.3;p=0.33) | −0.3(−0.6-0.1;p=0.25) | −0.3(−2.2-1.5;p=1.00) | −3.3(−6.3-0.2;p<0.05) |
| SM vs. R | ||||
| Borg Scale of PE,MD (95% CI) | −0.8(−1.7-0.0;p=0.05) | −0.6(−1.7-0.5;p=0.46) | −0.2(−1.2-0.9;p=1.00) | 0.7(−0.6-1.9;p=0.50) |
| Borg Dyspnea Scale,MD (95% CI) | 0.0(−0.4-0.5;p=1.00) | 0.1(−0.9-1.1;p=1.00) | 0.4(−0.5-1.3;p=0.63) | 0.8(−0.2-1.7;p=0.13) |
| SpO2 (%),MD (95% CI) | 0.0(−0.8-0.8;p=1.00) | 0.1(−0.9-1.0;p=1.00) | 0.0(−1.6-1.6;p=1.00) | −1.3(−3.2-0.7;p=0.30) |
Abbreviations: CI: confidence interval; ET: exercise testing; MD: mean difference; PE: perceived exertion; R: respirator; SM: surgical mask; SpO2: arterial oxygen saturation; WM: without mask.
A significant difference in level of dyspnea between testing conditions at 25%, 50% and at ET completion was found (Figure 1, Table 3). Using a R resulted in higher levels of dyspnea, compared to not wearing a mask, at 25%, 50% and at ET completion, while compared to WM, SM showed higher dyspnea levels only at 50% of total time achieved in ET. No differences were found in any stage of ET between wearing SM and R.
Progression of mean values of SpO2, level of perceived exertion (Borg Scale of Perceived Exertion) and level of dyspnea (Borg Dyspnea Scale) at different stages of the exercise test (at rest, at 25%, 50% and 75% of the duration of the test and at the end of the test). [SpO2: arterial oxygen saturation; ■: without mask; ●: surgical mask;
: respirator; *: signals p<0.05 on comparison of test conditions].Regarding SpO2, significant desaturation was only observed at ET completion when comparing R versus WM (Figure 1, Table 3). During cool down, at one and three minutes after ET completion, there were no significant differences between testing conditions ((mean(SD) at one minute: 96.0% (1.6) for those WM; 95.4% (1.6) for SM and 95.6% (2.0) for those wearing R; p=0.45); at 3 minutes: 96.4% (1.2) for those WM; 96.0% (1.2) for SM and 96.2% (1.0) for those wearing R; p=0.36)).
Hemodynamic response – heart rate and blood pressureHeart rate increased proportionally during each test, for each subject, according to the intensity of the exercise. When comparing HR at the end of the ET and chronotropic reserve, there were no significant differences between groups at any stage of ET (Table 2). The percentage of maximal age-predicted HR achieved at ET completion did not differ between groups with 90.0% (7.9) for WM, 90.9% (6.4) when wearing a SM and 89.8% (7.1) when wearing a R; there were no significant differences between these values. There were no statistically significant differences in mean systolic and diastolic end blood pressure according to testing conditions (p=0.30 and p=0.93, respectively). During cool down, at one and three minutes after ET completion, there were no significant differences in HR according to testing conditions ((mean(SD) at 1 minute: 147.3 (17.7) bpm for those WM; 144.1 (18.3) bpm for SM and 142.3 (20.9) bpm for those wearing R; p=0.56); at 3 minutes: 117.5 (19.6) bpm for those WM; 122.6 (19.2) bpm for SM and 120.0 (19.5) bpm for those wearing R; p=0.27)). No significant differences between test conditions for systolic BP at three minutes of recovery were found (p=0.31). Diastolic BP showed significant differences only between the test WM and with SM (p=0.04) – the mean (SD) values were 60.2 (5.9) mmHg, 69.0 (9.2) mmHg, and 66.0 (6.0) mmHg, respectively, for WM, SM and R.
DiscussionThe main findings of our study were as follows: (1) regardless of type of mask worn, using a FM resulted in shorter duration of maximal symptom-limited ET and higher levels of dyspnea and perceived exertion; (2) a significant drop in SpO2 was present at the end of ET only when using a R; (3) there were no differences in either chronotropic response or BP between testing conditions; (4) there were no significant differences in response to ET between wearing SM or R. In the light of the restrictions imposed on cardiac rehabilitation by COVID-19, including regarding safety in group exercise sessions, there have been a few recent studies on the impact of wearing different types of FM on cardiorespiratory parameters and subjective levels of dyspnea and exertion during exercise,9,15–17 albeit showing mixed results. Fikenzer et al.15 found that cardiopulmonary exercise capacity is reduced by SM and highly impaired by R during a progressive cycle ergometer test. In contrast, Epstein et al.17 found only minor changes in physiological parameters during a progressive cycle ergometer test when wearing a SM or a R, and Shaw et al.9 did not report any discernable detrimental effect on exercise performance while using a FM during a cycle ergometer test onexhaustion.
Our study showed a shorter duration of ET when using a FM, regardless of its type, reflecting limitations at higher levels of exercise intensity. SM and R might exert these detrimental effects on exercise capacity through different mechanisms: Resistance to airflow and heightened respiratory pressures,18 increased dead space ventilation,19 alveolar hypoventilation20,21 and interference with tidal volume, thermal regulation, vision, communication and task performance.19 It is known that FM restrict airflow and require generation of higher inspiratory and expiratory pressures when breathing,20,22 changing the inspiration and expiration process from passive to active18 and leading to increased breathing work, respiratory muscle fatigue22,23 and reduced exercise performance.24 Considering that perceived breathing effort is positively correlated with ventilation effort,25 the increased respiratory load may itself be associated with physical and psychological discomfort. On the other hand, higher resistance to airflow results in alveolar hypoventilation,20,21 leading to an earlier onset of the first and second ventilatory thresholds and a shorter time to exhaustion and achievement of lower peak VO2.8,26 There is also a substantial variability in the individual tolerance19 of higher respiratory resistance and to the hot and humid conditions created inside the FM.27 The increase in temperature of inhaled air associated with wearing a FM might also result in bronchoconstriction and higher pulmonary resistance,28 contributing further to dyspnea and respiratory discomfort.29 This tolerance is also greatly influenced by the anxiety levels of the wearer.30
Granados et al.,31 in a pilot study to assess the impact of FM on exercise performance and ventilatory responses, postulated that, for effort levels above 60% VO2peak, wearing a FM was associated with inadequate hyperventilation and arterial hypoxemia due to reduced breathing frequency and dead space carbon dioxide rebreathing. Accordingly, our study shows a reduction in SpO2 measured during ET when using a SM or a R, although the difference was only significant during maximal exercise intensities using a R, comparing to not wearing a FM. In contrast, other studies found no significant impact on SpO2 when wearing a SM during a submaximal exertion measured during the six-minute walk test,32 a SM or a R during a cycle ergometry test of time to exhaustion9,17 or when wearing a R during a low to moderate intensity walk (5.6 km/h) on a treadmill for an hour.12 We believe that respiratory function (gas exchange) may not represent a limiting factor for peripheral oxygen carrying capacity during mild to moderate intensity exercise in healthy adults, despite the loaded breathing.
Perceived exertion has an array of possible determinants, ranging from physiological, volitional, motivational and psychological.33 In the present study, compared to not using any FM, perceived exertion was higher when using either a SM or a R; notably, it was most significant at the first stages of ET, when using a SM, and most significant toward the end of it when using a R. On the other hand, perceived breathing effort was significantly higher when using a R throughout the ET, compared to not wearing a FM. Perceived breathing effort with the SM was only significantly higher than WM at half of the ET duration. Both hypoxemia and respiratory muscle fatigue, resulting from increased work of breathing because of increased airflow resistance, possibly contribute to increased perceived exertion at maximal exercise intensity. This trend is supported by Johnson et al.,21 who demonstrated that the impact of inspiratory resistance on performance is mostly felt at higher exercise intensities. Fikenzer et al.15 also demonstrated higher perceived exertion when wearing a SM or a R during a maximal symptom-limited cycle ergometer test, findings that contradict a study by Roberge et al.23 on the effects of wearing a surgical mask during a treadmill exercise test for one hour. In this study, there was no significant impact on perceived exertion. Shaw et al.9 also did not report any detrimental effect of FM on perceived exertion.
There is an alternative view on the beneficial effects of wearing a FM, especially R, during exercise training programs. Davis and Tsen34 postulate that the increased work of breathing derived from using this type of FM during exercise might be used for conditioning purposes, increasing respiratory muscle strength and respiratory muscle endurance, improving ventilatory efficiency, oxygen delivery and overall exercise performance. This is of particular importance in the context of CRP, considering the overall objectives of this intervention.
Loading in breathing effort during exercise may reduce respiratory frequency and implies an additional muscle recruitment and potentially slow oxygen consumption kinetics,32 a myriad of factors that may possibly change the signaling in the central CV center and increase HR and BP.35 Based on that, the authors hypothesized that exercising while wearing a FM would greatly influence hemodynamics when comparing to not wearing a FM, as demonstrated on healthy volunteers performing a low to moderate intensity walk (5.6 km/h) on a treadmill for an hour.16 However, our study did not demonstrate a significant difference in BP and HR response during and after ET in the different testing conditions besides a shorter time to reach maximal HR values. Jung et al.36 reported similar results in their study on the effect of an elevation training mask during cycling – although they reported a significant autonomic-mediated blunted HR decrease during recovery when wearing the mask. Data from multiple studies have also shown that the use of a FM has no significant effect on HR.12,24,37 Nevertheless, the authors believe that further studies are necessary to draw more definite conclusions on the CV response to wearing a FM during exercise, especially in those patients with previous CV and pulmonary disorders.
Based on other studies,36,37 our findings may be related to the combination of duration and intensity of the exercise performed. Interestingly, after an initial period of subjective destabilization during the first half of the ET when using a FM, there seems to be a leveling of the perceived discomfort at mid-stage of the ET (Figure 1). This potential compensation mechanism appears to become ineffective as the subject reaches their maximal exercise capacity, possibly due to the breathing difficulty resulting from an increase minute-ventilation against an increased inspiratory and expiratory resistance from wearing the FM. In type 2 SM, this resistance is usually expressed as a differential pressure of 40 Pa/cm2.38,39 On the other hand, KN95 Rs show a maximal differential pressure of approximately 70 Pa/cm2 for inhalation flow and 50 Pa/cm2 for exhalation flow, considering a flow rate of 85L/min.13,38 These values increase in proportion to the flow rate, which depends on the ventilation per minute. For this reason, it is reasonable that perceived exertion and dyspnea are significantly higher with either FM throughout the ET, when compared to not wearing a FM. At lower intensities, the air resistance offered by SM and R is low and probably similar, as flow rates are still increasing. However, at maximal intensity, when flow rates and air resistance from using FM are at their highest, there is a steep increase in work of breathing resulting in fatigue of respiratory muscles, higher perceived exertion and dyspnea, especially when wearing a R.
In the setting of CRP, professionals should be aware of the physical and psychological discomfort caused by FM, as well as be advised of a possible influence on CV response. Relative oxygen desaturation is a major concern in this patient population, especially when there is concomitant respiratory pathology. Patient familiarity with the physiological adjustments that occur when using a FM can lead to enhanced effectiveness of therapeutic exercise in this context. Nevertheless, the authors believe that implementation of monitored home-based CRP may replace in-hospital sessions in some cases, particularly in low CV risk patients, as has already been suggested.5 It seems reasonable to start with remote low-intensity exercise training in combination with resistance and flexibility exercises after a safety assessment. These home-based programs may be monitored through tele-rehabilitation and circumvent the need of using a FM during exercise.40
LimitationsSome limitations must be considered. This is a preliminary single-center study of a limited number of overall healthy, relatively young health workers, who agreed to participate. Caution is recommended when extrapolating results to clinical settings and different populations, namely older patients, those with high comorbidity burden and patients attending CV or pulmonary rehabilitation exercise programs. On the other hand, we must consider that the subjects in this study are healthcare workers who routinely use a FM for long periods of time – this can impact on their tolerance of the equipment compared with other subjects. Additionally, the lack of multivariable analysis according to age, gender and physical activity habits also limits the generalization of these results. Although the investigators followed a strict protocol of ET, randomization of ET sequence, blinding of researchers involved in data abstraction and database completion, difficulties from nonblinding of patient and researcher performing the ET could not be overcome. Moreover, no information was collected regarding other ventilatory parameters, including direct measurement of dead space with different FM, minute-ventilation and/or respiratory frequency, baseline cardiorespiratory performance (cardiopulmonary ET).31
ConclusionsOur study found that wearing a FM may be associated with reduced exercise capacity and higher levels of dyspnea and effort perception, especially at maximal intensity, independent of the type of FM worn. There is no evidence that wearing a FM during high intensity aerobic exercise significantly changes chronotropic and BP responses. On the other hand, wearing a R in these conditions induces arterial hypoxemia in healthy adults. In the setting of CRP, these findings may represent a major concern during the COVID-19 pandemic and may warrant a readjustment of the exercise intensities used in this context. To further assess and in order to make exercise prescription recommendations, further trials are needed, including with larger and more representative samples and with more extensive indicators of cardiopulmonary responses to wearing different types of FM.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to thank to João Barroso Monteiro, MD, Department Director of Physical Medicine & Rehabilitation at Centro Hospitalar e Universitário de São João, for his scientific guidance and manuscript revision.





![Progression of mean values of SpO2, level of perceived exertion (Borg Scale of Perceived Exertion) and level of dyspnea (Borg Dyspnea Scale) at different stages of the exercise test (at rest, at 25%, 50% and 75% of the duration of the test and at the end of the test). [SpO2: arterial oxygen saturation; ■: without mask; ●: surgical mask; : respirator; *: signals p<0.05 on comparison of test conditions]. Progression of mean values of SpO2, level of perceived exertion (Borg Scale of Perceived Exertion) and level of dyspnea (Borg Dyspnea Scale) at different stages of the exercise test (at rest, at 25%, 50% and 75% of the duration of the test and at the end of the test). [SpO2: arterial oxygen saturation; ■: without mask; ●: surgical mask; : respirator; *: signals p<0.05 on comparison of test conditions].](https://static.elsevier.es/multimedia/21742049/0000004000000012/v1_202112160943/S2174204921003676/v1_202112160943/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)


