Acute heart failure (HF) decompensation generally manifests with signs and symptoms of congestion that strongly predict poor poor patient outcome. Loop diuretics are the cornerstone of therapy to counteract fluid overload and are widely used for acute management and chronic stabilization of HF. However, a diminished response to loop diuretics is a common problem, affecting the patient's clinical course and potentially prolonging hospitalization. Diuretic resistance is defined as failure to decongest despite appropriate and escalating loop diuretic therapy. We propose a protocol for the management of diuretic resistance. The initial approach should include an assessment of causes of pseudo-diuretic resistance. Adjustments to loop diuretic therapy, such as increasing doses and frequency of administration and sequential nephron blockade, may be successful. For hospitalized patients with progressive disease there are more invasive methods for fluid removal. Switching from oral to intravenous loop diuretics is essential to avoid variable absorption and for symptomatic relief. Extracorporeal ultrafiltration is also an option since this technique is highly effective at removing plasma fluid from blood. While extracorporeal ultrafiltration is an invasive solution, peritoneal dialysis is a home-based, intermittent therapeutic option that can enable efficient management of fluid overload, preventing HF-related hospital admission, and improving quality of life. As a last resort for fluid removal, a peritoneal dialysis regimen should fully exploit its decongestive properties and should be tailored to the patient's characteristics and clinical needs.
A insuficiência cardíaca (IC) descompensada manifesta-se por sinais e sintomas de congestão, que são fortes preditores de mau prognóstico. Os diuréticos de ansa são a base do tratamento da sobrecarga hídrica nas descompensações agudas e na estabilização crónica, mas uma resposta diminuída aos mesmos é frequente na IC. Tal afeta o curso clínico e condicionais internamentos mais prolongados. A «resistência a diuréticos» é definida pela incapacidade em tratar sintomas congestivos, apesar da terapêutica adequada e crescente com diuréticos de ansa. Propomos um protocolo de atuação para ultrapassar esta resistência em que a abordagem inicial passa pela exclusão de causas de pseudo-resistência. Estratégias como o aumento da dose ou do número de administrações de diuréticos de ansa, ou o bloqueio sequencial do nefrónio, podem ser suficientes para aumentar a resposta diurética. Em doentes hospitalizados existem métodos mais invasivos para a remoção de fluidos. Nestes, a alteração de um diurético de ansa oral para endovenoso é uma abordagem fundamental para evitar a absorção errática e para o alívio sintomático. A ultrafiltração extracorporal é uma técnica invasiva muito eficaz na remoção de fluidos, que também pode ser uma opção terapêutica. A diálise peritoneal é um tratamento domiciliar intermitente que permite uma gestão eficiente da sobrecarga hídrica, evitando internamentos por IC descompensada e garantindo uma melhor qualidade de vida. A prescrição de diálise peritoneal para a remoção de fluidos, num doente sem resposta a outras medidas médicas, deve otimizar ao máximo a sua capacidade de ultrafiltração e deve ser adaptada às características do doente.
Heart failure (HF) is a prevalent condition worldwide with high health care costs due to its disabling clinical behavior and associated recurrent hospital admissions, especially in the elderly.1,2 Acute HF decompensation generally manifests with signs and symptoms of congestion that strongly predict poor patient outcome.3,4 Overall, the kidneys, being the major effectors of compensatory activity, play a central role in HF-related congestion and consequently management of HF symptoms.5 Despite the lack of evidence of a mortality benefit in HF,6–8 efficacious diuretic therapy has been shown to prolong event-free survival and to relieve symptoms of congestion.9 Loop diuretics are the cornerstone of this therapy and are widely used for acute management and chronic stabilization of HF.10 However, a common problem is a diminished response to loop diuretics, affecting the patient's clinical course and potentially prolonging hospitalization. The term diuretic resistance (DR) is still not clearly defined,11 but is generally taken to mean failure to decongest despite appropriate and escalating loop diuretic therapy.10–12 A poor diuretic response may be measured in different ways, depending on the study.13–15 In this analysis, we define an insufficient diuretic response in cases of congestion with volume overload as a spot urine sodium content <50–70 mEq/l after 2 h, and/or an hourly urine output <100–150 ml during the first 6 h after the administration of the drug.16
Risk factors for DR are better established and include a fractional excretion of sodium at baseline of less than 0.2%,17 chloride levels at baseline between 97 and 103 mEq/l,18 diabetes, low systemic blood pressure, elevated blood urea nitrogen and HF of ischemic origin.10 Physiologic alterations in acute HF can alter loop diuretic pharmacokinetics and lead to DR, such as decreased gastric emptying, reduced splanchnic blood flow, intestinal wall edema19,20 and impaired delivery or reduced active secretion of loop diuretics by the organic acid transporter into the proximal tubule, as a consequence of HF-related decreased glomerular filtration.21 However, an increase in dose or alteration in administration route should overcome this resistance if pharmacokinetic alterations were solely responsible for DR. A combination of pharmacokinetic and pharmacodynamic changes affecting the time course of diuretic delivery may explain the lack of therapeutic response.22
Management of diuretic resistanceAs long as the concentration of a loop diuretic in tubular fluid is sufficiently high to block the Na+/K+/2Cl− cotransporter, there is a natriuretic response that activates the renin-angiotensin-aldosterone system (RAAS), leading to increased sodium absorption along the distal nephron. As soon as diuretic concentration falls below the diuretic threshold, these compensatory mechanisms trigger post-diuretic sodium retention. This involves all tubular segments and is enhanced when drug-free intervals are longer than four half-lives of the loop diuretic. If sodium intake is high, a negative sodium balance may not be achieved because post-diuretic salt retention can completely abolish the effect of the diuretic.23–25 After a chronic period of loop diuretic administration, enhanced sodium delivery to the distal tubular system results in compensatory hyperplasia and hypertrophy. Consequently, after multiple same-dose administration of diuretics, diuresis volume decreases, which is called the braking phenomenon.10,26
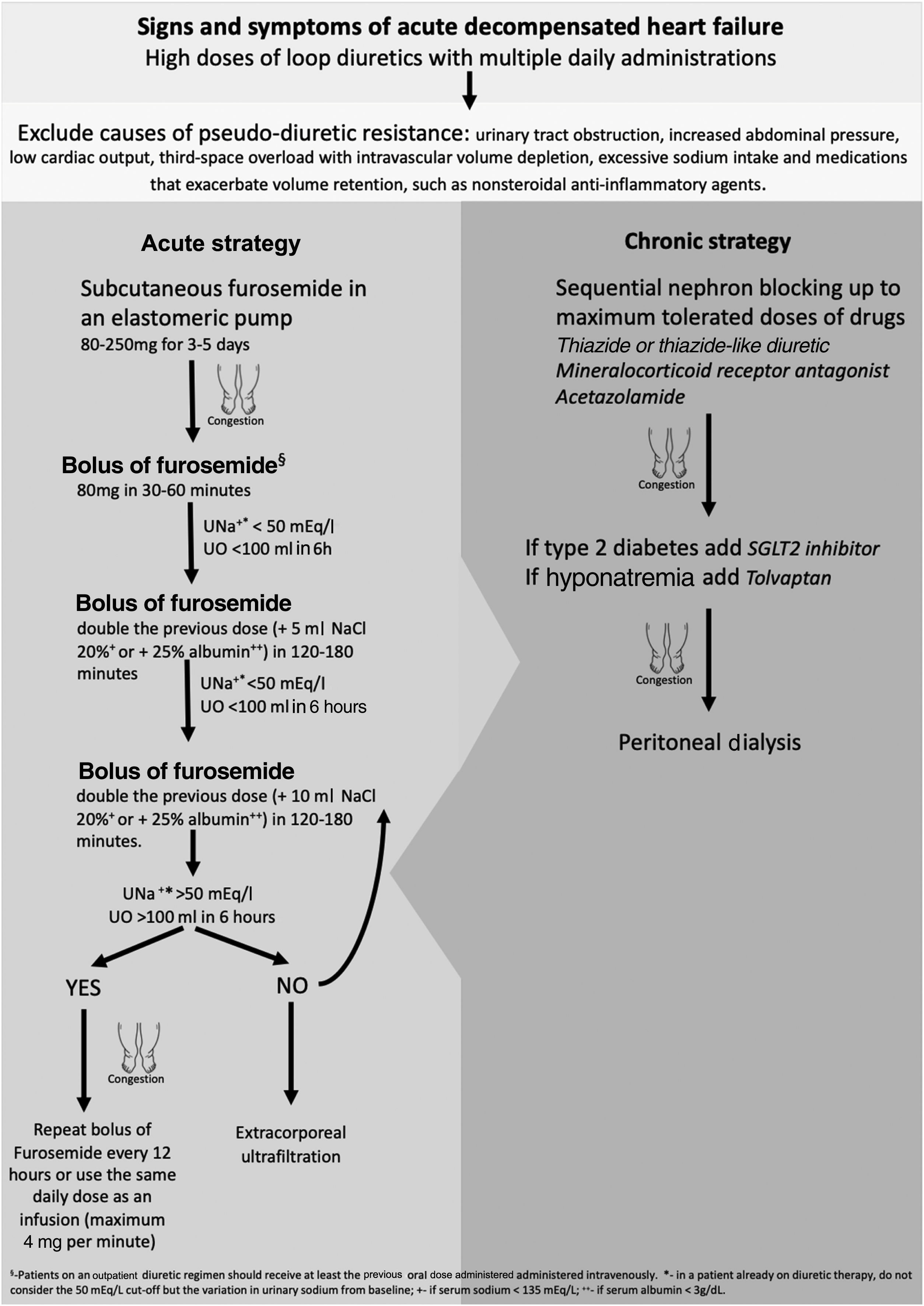
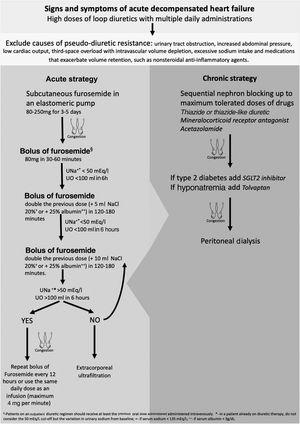
DR for any reason becomes a challenging problem in the hospital management of acute HF decompensation for patients treated with oral medication but with progressively worsening congestive symptoms. The initial approach to acute decompensated HF with DR should include an assessment of causes of pseudo-diuretic resistance, such as third-space overload with intravascular volume depletion (Figure 1).10
It is important to ensure that sodium intake is less than 100 mEq/day in order to mitigate the effects of post-diuretic sodium retention and to achieve a negative sodium balance.27 The complete list of the patient's medications should also be reviewed, to ensure adherence and to identify drugs that exacerbate volume retention, such as nonsteroidal anti-inflammatory agents, and discontinue or replace them with safer alternatives.28 If DR persists, adjustments to loop diuretic therapy may be considered. It is often necessary to increase the dose of a loop diuretic: 250–4000 mg/day, given orally, has been shown to relieve symptoms and reduce weight with no significant side effects.29 Furthermore, more frequent administration of the diuretic can overcome the effects of post-diuretic salt retention by reducing the drug-free interval, and hence restore diuretic response.30
In the context of the SARS-CoV-2 pandemic, there have recently been efforts to reduce HF hospital management and its costs. Continuous subcutaneous furosemide administration by elastomeric pump enables congestion to be treated on an outpatient basis.31,32 This approach can be beneficial for all patients whose symptoms are uncontrolled, who do not respond to oral therapy, and/or who are not indicated for hospitalization. The acute strategy of our protocol is more often initiated at a heart failure day hospital but can also be used for hospital admissions, especially for elderly and frail patients in whom vascular access is more difficult and painful. Respiratory failure is the criterion for compulsorily hospitalizing the patient.33
Another method to overcome DR is sequential nephron blockade. Adding alternative classes of diuretics provides synergistic effects by avoiding rebound sodium retention. Acetazolamide inhibits proximal sodium reabsorption causing increased sodium concentrations in the loop of Henle, in which loop diuretics exert their natriuretic action. However, only short-term use is suggested due to the risk of metabolic acidosis. Another approach is to block sodium reabsorption at the distal tubule by adding a thiazide or thiazide-like diuretic. Distal tubule diuretics also prevent the adaptive process of cell hypertrophy that occurs with long-term loop diuretic use.34,35 The two most commonly used thiazides are metolazone and hydrochlorothiazide. Metolazone may be favored over other thiazide-type diuretics because of its additional effect on the proximal tubule, high oral bioavailability and low cost.36 However, there is no clear evidence of superiority in terms of increasing diuresis, maintenance of renal function or avoiding electrolyte abnormalities.10 Some authors recommend administering thiazides before loop diuretics to allow time for full blockade of the distal nephron.27 As thiazide-type diuretics limit the kidneys’ capacity to dilute urine, they reduce free water clearance and should therefore be avoided in hypotonic hyponatremia. Other side effects are hypokalemia, dehydration, and renal failure.37 Most patients with HF are already on a low dose of a mineralocorticoid receptor antagonist because of these drugs’ beneficial effect on mortality and HF hospitalizations. They are recommended in all cases of acute decompensated HF and reduced ejection fraction and should be added to treatment in naïve patients.38 It is not clear whether there is a synergistic diuretic effect between mineralocorticoid receptor antagonists and loop diuretics.39,40 Besides minimizing potassium wasting by loop diuretics, spironolactone, eplerenone and finerenone have a mild but effective natriuretic effect. The rationale for using these drugs may not actually be sequential nephron blockade, but there is evidence for long-term benefit with increased dosages if glomerular filtration rate is stable and in the absence of hyperkalemia.6,37
Tolvaptan inhibits vasopressin by binding to vasopressin receptors, preventing AQP2 recruitment and thereby leading to free-water excretion.41 It results in short-term weight loss and dyspnea improvement in worsening HF, and can be a good adjunctive therapy in patients with DR.42
For hospitalized patients, switching to intravenous (IV) loop diuretics is a way to avoid variable oral absorption and for symptomatic relief.43 An intravenous dose between 400 and 600 mg of furosemide is generally considered the maximum total daily dose recommended, above which limited additional natriuresis should be expected but side effects will continue to increase.16
Whether to select intermittent bolus or continuous IV administration as an initial strategy remains controversial. Some studies conclude that IV boluses and continuous infusion have similar effectiveness and safety profiles,44 while others claim that there are potential advantages to continuous infusion administration as this eliminates post-diuretic sodium retention and permits higher daily doses.31,32 We suggest a bolus of 80 mg or higher of IV furosemide in patients with reduced glomerular filtration rate. Patients on an outpatient diuretic regimen should receive at least the pre-existing oral dose administered intravenously. The DOSE-AHF trial demonstrated that high loop diuretic dose (2.5 times the usual home dose, with at least 80 mg/day of furosemide) compared to low dose (equal to home dose) resulted in a favorable effect on the secondary endpoints of dyspnea relief, change in weight and net fluid loss.44 Hypertonic saline solution administered in conjunction with furosemide improves diuretic and sodium excretion rate. Some studies have also shown an all-cause mortality benefit, hypothesized to be due to decreased activation of adrenergic and renin-angiotensin systems by sodium overload and a consequent decrease in their detrimental effects on the cardiovascular system, improving outcome beyond the associated diuresis. This therapy is especially indicated for patients with hyponatremia as it causes a significant rise in serum sodium.45
Albumin infusion in addition to parenteral loop diuretics in hypoalbuminemic patients with acute HF showed improved diuresis and return to dry weight. The pathophysiologic mechanism remains unclear, but benefits are usually attributed to an increase in plasma oncotic pressure and increased diuretic delivery to the nephron.46
Extracorporeal ultrafiltration (ECUF) is also an option. This technique is highly effective at removing plasma fluid from blood across a semipermeable membrane through a hydrostatic pressure gradient. ECUF has been hypothesized to possess several advantages over diuretic therapy, including greater weight loss and fluid removal without affecting renal function, and lower mortality and rehospitalization, but without consistent results.47–52 Although ECUF is an excellent technique for volume control in diuretic resistant patients, it is an acute therapy for symptomatic relief and hemodynamic stabilization, in the absence of end-stage renal disease. For long-term control of hypervolemia, its efficacy and safety are not well established, and in addition it is a hospital-based therapy, which leads to a significant decrease in patients’ quality of life.10,11 The main goal in HF refractory to diuretics is to control volume on an outpatient basis, without decompensation or hospitalizations.
While ECUF is a hospital-based solution, peritoneal dialysis (PD) is a home-based intermittent therapeutic option. PD is usually prescribed as a renal replacement therapy in end-stage kidney disease, but it can be customized to varying patient clinical needs. The rationale for using PD as a method of fluid removal in congestive HF is manifold. It also allows effective solute clearance, which leads to better uptitration of HF drugs such as RAAS blockers, and to enhanced diuretic responsiveness.53,54 Often after IV diuretics or ECUF there is neurohumoral activation due to intravascular volume depletion. Because peritoneal ultrafiltration has a minimal impact on hemodynamics, there is no sympathetic and RAAS response, which results in better preservation of residual renal function.55 Peritoneal cavity access allows for drainage of ascitic fluid in cases of right-sided heart failure, reducing intra-abdominal pressure, which also improves renal function in HF.56 There is also speculation that PD removes other harmful mediators such as tumor necrosis factor-alpha, interleukin-6 and myocardial depressant factor.57,58 Compared to ECUF, PD is not associated with myocardial stunning or vascular access complications such as catheter-related bloodstream infections, or negative effects on cardiac function due to high-flow arteriovenous fistulas.53 Other advantages of PD are that it can be placed under local anesthesia, without needing to discontinue anticoagulation and antiplatelet therapy and can be used immediately, the ability to achieve overnight peritoneal ultrafiltration in patients with hernias without the need for hernia repair, and the low rate of complications such as peritonitis and catheter dysfunction.59,60 PD has also been shown to improve New York Heart Association functional class, reduce hospitalizations and mortality, and improve quality of life, in decompensated HF with or without mild kidney failure associated with type 2 cardiorenal syndrome (CRS).61–63
Depending on the patient's need, a PD prescription can specifically target fluid removal, solute clearance, or both. In the setting of congestive HF, the primary focus with PD should be optimization of sodium removal, as sodium is the major determinant of extracellular volume. In order to achieve better volume management, sodium removal can be optimized in a dwell by eliminating the early phase, sodium sieving, using icodextrin. Fluid removal can be achieved with a single nocturnal peritoneal exchange with icodextrin in refractory diuretic-resistant HF.64 With progression of CRS over time, the PD regimen can be gradually intensified by adding glucose-based solutions to provide more clearance and sodium removal. Continuous ambulatory PD has less frequent and longer cycles than automated PD (APD) and theoretically results in higher sodium removal. However, it is believed that with optimal PD prescription, the two modalities have similar results. Adding a manual daytime exchange to an APD regimen is another option to enhance sodium elimination.65 In HF patients with hyponatremia, more frequent short dwells of hypertonic dialysate solutions can lead to high clearance of free water. Twice-daily 8-h icodextrin exchanges seem to be a promising approach for efficient decongestion in refractory HF. Some studies have shown an increase in ultrafiltration volume and ejection fraction as well as reduction in weight, blood pressure, N-terminal pro-brain natriuretic peptide, and left ventricular mass. However, for long-term use, close monitoring of serum sodium levels is recommended.66,67
ConclusionIn HF there is a compensatory activation of neurohormonal systems to maintain circulatory homeostasis, which leads to fluid overload. Diuretic therapy is still the cornerstone of therapy to prevent and treat congestion; however, with long-term treatment there is a decrease in its therapeutic effect. DR has emerged as an independent factor for worse outcomes in HF patients. There are some non-pharmacological measures and a few medical options to overcome DR. Adjustments to loop diuretic therapy, such as increasing doses and frequency of administration and sequential nephron blockade, may be successful. For hospitalized patients with progressive disease there are more invasive methods for fluid removal. PD can enable efficient management of fluid overload, preventing HF-related hospital admission, and improving quality of life. As a last resort for fluid removal, a PD regimen should fully exploit its decongestive properties and should be tailored to the patient's characteristics and clinical needs.
Conflicts of interestThe authors have no conflicts of interest to declare.