Anticoagulation control as assessed by time in therapeutic range (TTR) correlates positively with the safety and efficacy of thromboprophylaxis in atrial fibrillation. We set out to assess TTR in our unit and to investigate determinants of better control.
MethodsThis was a case series study of atrial fibrillation patients anticoagulated with warfarin or acenocoumarol at the Family Health Unit of Fânzeres. Sociodemographic and clinical data were collected and TTR was calculated by the Rosendaal method, based on international normalized ratio tests performed in external laboratories in the preceding six months. SPSS® 21.0 was used for the statistical analysis, with descriptive statistics, Spearman's correlation, and the Mann-Whitney U and Kruskal-Wallis tests.
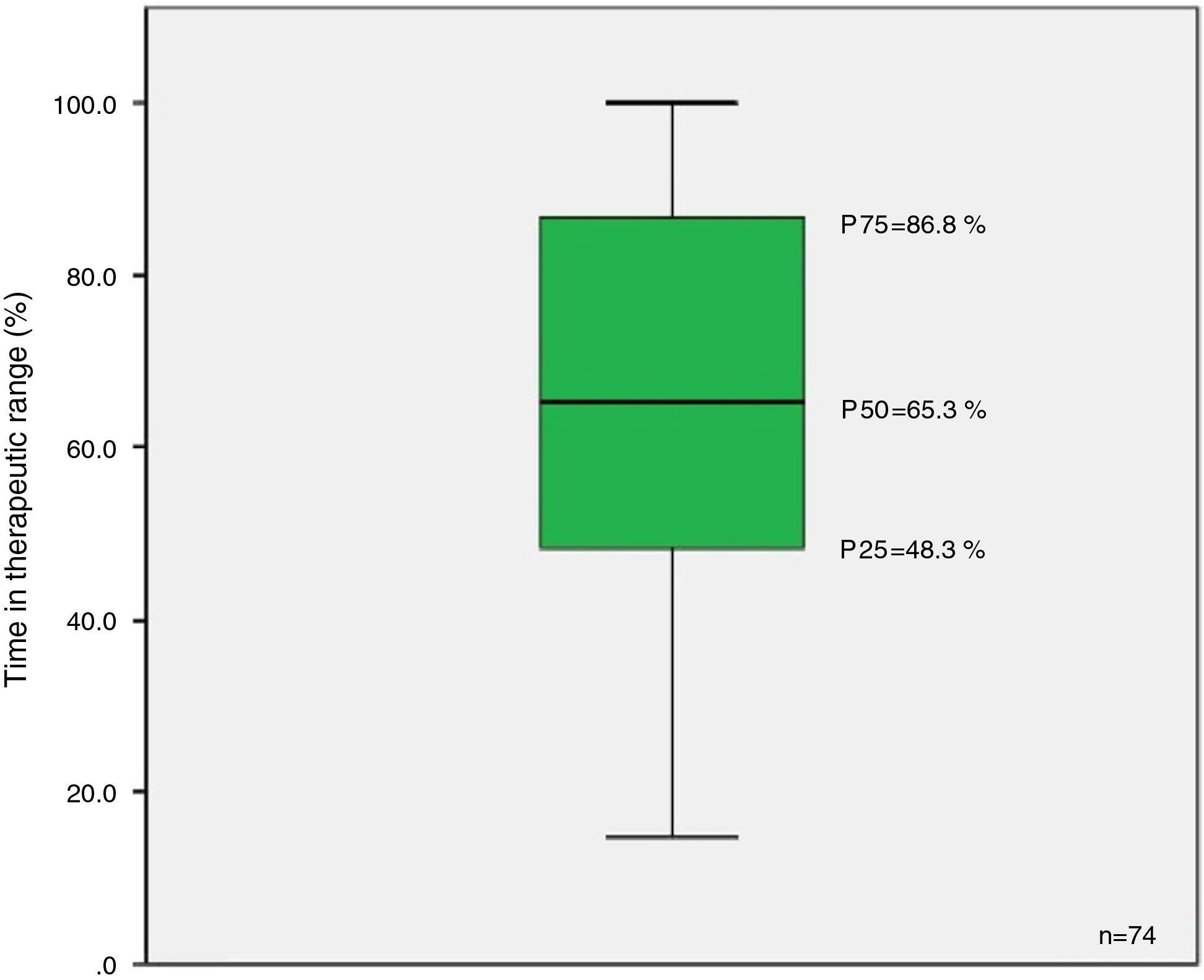
ResultsOf the 106 eligible patients, 70% participated in the study. Median TTR was 65.3% (P25=48.3%, P75=86.8%). We found a positive association between this variable and duration of atrial fibrillation (rho=0.477, p<0.001, r2=0.116) and with duration of anticoagulation (rho=0.5, p<0.001, r2=0.087). No association was found with age, gender, educational level or existence of a caregiver (p>0.05).
ConclusionsMedian TTR in our unit is similar to that in southern European countries and close to the good control threshold (70%) proposed by the European Society of Cardiology. The duration of atrial fibrillation and of anticoagulation explains only a small part of the measure's variability. Other determinants of anticoagulation control must be investigated in future studies and comparative studies should be carried out in family health units monitoring anticoagulation on the premises.
Na fibrilhação auricular o controlo da hipocoagulação avaliado pelo tempo em alvo terapêutico correlaciona-se positivamente com a segurança e eficácia tromboprofiláticas. Pretende-se realizar essa avaliação na unidade dos autores e pesquisar determinantes de melhor controlo.
MétodosSérie de casos – utentes da Unidade de Saúde Familiar de Fânzeres com fibrilhação auricular sob varfarina ou acenocumarol. Colheram-se dados sócio-demográficos e clínicos; calculou-se o tempo em alvo terapêutico pelo método de Rosendaal, usando os resultados de Razão Normalizada Internacional realizados em laboratórios externos à unidade nos seis meses precedentes. Os dados foram tratados em SPSS 21.0®, recorrendo-se a estatística descritiva, correlação de Spearman, teste de Mann-Whitney U e Kruskal-Wallis.
ResultadosParticiparam 70% dos 106 pacientes elegíveis. O tempo em alvo terapêutico mediano foi de 65,3% (P25 = 48,3%; P75 = 86,8%). Encontrou-se relação positiva entre essa variável e duração da doença (ρ = 0,477; p < 0,001; R2 = 0,116) e duração da hipocoagulação (ρ = 0,5; p < 0,001; R2 = 0,087). Não se estabeleceu relação com idade, género, escolaridade e existência de cuidador (p > 0,05).
ConclusõesO tempo em alvo terapêutico mediano na unidade é semelhante ao dos países sul europeus, ficando próximo do «bom controlo» definido pela Sociedade Europeia de Cardiologia (70%). A duração da fibrilhação auricular e da hipocoagulação explicam uma pequena parte da variabilidade dessa medida. Serão necessários estudos para identificação de outros determinantes do controlo da hipocoagulação e estudos comparativos com unidades que realizem os testes laboratoriais de monitorização da hipocoagulação nas suas instalações.
atrial fibrillation
Family Health Unit
international normalized ratio
Database and monitoring software for Primary Care Units in the Portuguese national health system
oral anticoagulant
25th percentile
50th percentile
75th percentile
time in therapeutic range
Atrial fibrillation (AF) is the most common sustained supraventricular tachyarrhythmia encountered in clinical practice, with a prevalence on electrocardiographic studies of 2.5% in the Portuguese population aged 40 or over, 36% of whom are undiagnosed.1 Prevalence increases with age, reaching 10% in those aged 80 or over.1
If conversion to sinus rhythm is not indicated or possible, AF becomes chronic, which often entails changes in lifestyle and thromboprophylaxis with oral anticoagulants (OACs) that require laborious dose adjustment. Poor adherence to therapy places patients at high risk for morbidity and mortality, particularly from stroke.1–7 The classic OACs are warfarin and acenocoumarol, for which the target international normalized ratio (INR) is between 2.0 and 3.0, based on their proven efficacy for prevention of stroke and pulmonary embolism, together with an acceptable safety profile in terms of bleeding risk.7,8
The time in therapeutic range (TTR), expressed as a percentage, is a measure of anticoagulation control, ≥70% being proposed as good control by the European Society of Cardiology.7 This is reflected in greater efficacy and fewer adverse effects: an 12% improvement in TTR is associated with a reduction in thromboembolic events of 1 event per 100 patient-years, while a 7% increase is associated with a reduction in major bleeding of 1 event per 100 patient-years.8
Data from the literature show differences between regions and countries, with TTR ranging from 50% in Israel to 64% in southern Europe, 74.5% in northern Europe and 76.5% in Sweden.8–16 The Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial, the only source of data on TTR in Portugal, reports 61% for participants anticoagulated with warfarin.17 However, this was a phase III trial comparing the efficacy and safety of warfarin and a new OAC, dabigatran, for stroke prevention in AF patients, and the figure reported thus reflects the specific conditions of the trial, which are not necessarily comparable to everyday clinical practice. There are two other studies on anticoagulation control in Portugal, but these report the percentage of INR values within the therapeutic range in specific health units.18,19 This measure provides different information from TTR, which focuses on individual patients; these studies are thus not comparable to the present one.
Furthermore, there are differences in setting and level of care in monitoring anticoagulation. A meta-analysis of US patients with AF under warfarin therapy revealed that those followed in specialist units presented higher TTR than those followed in primary care (63% vs. 51%, respectively).20
Since there are no data on TTR in everyday clinical practice in primary care centers in Portugal, particularly when anticoagulation levels are assessed by external laboratories, we decided to assess TTR in AF patients anticoagulated with classic drugs in the Family Health Unit (FHU) in the town of Fânzeres.
ObjectivesThe aims of the study were:
- (1)
to analyze the relationship between TTR and sociodemographic variables (age, gender, educational level and existence of a caregiver) and clinical variables (duration of AF and of anticoagulation); and
- (2)
to characterize TTR in AF patients at the Fânzeres FHU anticoagulated with classic drugs (warfarin or acenocoumarol).
This was a case series study, conducted between May and August 2013, of Fânzeres FHU patients with a diagnosis of AF (paroxysmal, persistent or permanent) under classic OACs (warfarin or acenocoumarol).
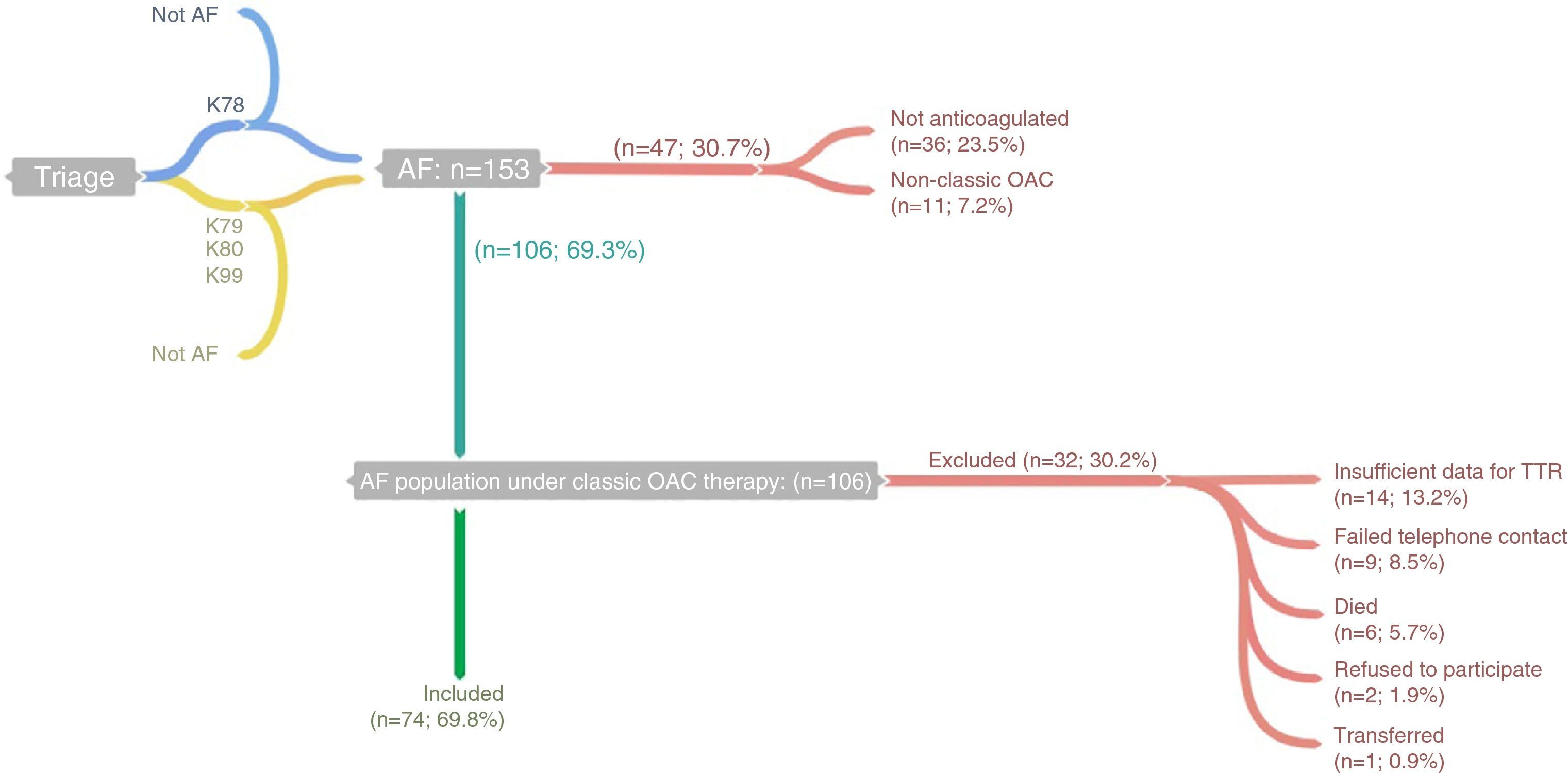
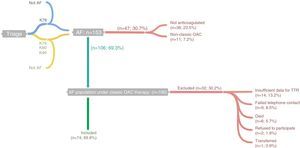
The study population was selected from the lists of Fânzeres FHU physicians’ patients with a diagnosis of AF, classified in accordance with the International Classification of Primary Care, 2nd edition (ICPC-2) as code K78 (Atrial fibrillation/flutter), as at May 31, 2013. Patient lists were obtained from MIM@UF (database and monitoring software for Primary Care Units in the Portuguese national health system). In addition, we examined the lists of patients classified with code K79 (Paroxysmal tachycardia), K80 (Cardiac arrhythmia NOS) and K99 (Cardiovascular disease other) (Figure 1), at May 31, 2013. The individual records of the patients listed (clinical records and diagnostic exams) were examined for clinical information on a diagnosis of AF (medical assessment or electrocardiogram documenting AF), and 153 individuals were identified.
Flowchart showing identification of cases and inclusion/exclusion criteria. K78, K79, K80 and K99 refer to “Atrial fibrillation/flutter”, “Paroxysmal tachycardia”, “Cardiac arrhythmia NOS” and “Cardiovascular disease other”, respectively, in accordance with the International Classification of Primary Care, 2nd edition; this information was obtained through MIM@UF. AF: atrial fibrillation; OAC: oral anticoagulant; TTR: time in therapeutic range.
In order to identify patients under therapy with warfarin or acenocoumarol in the previous months, prescriptions were examined and patients were contacted by telephone (up to five attempts at different times and on different weekdays). By preference, contact was made with the patients themselves, but when necessary with their caregiver or legal guardian (defined as the person responsible for administering the patient's medication). Forty-seven individuals were excluded as they were not taking warfarin or acenocoumarol.
Of the 106 patients anticoagulated with the classic drugs eligible for the study, six died during 2013, one was transferred to a different FHU, nine could not be contacted after the five attempts stipulated in the protocol, and two refused to participate. After patient agreement to enrollment in the study, a personal interview was scheduled at the FHU.
Monitoring of OAC therapy in Fânzeres FHU patients is performed by laboratories in the community or by hospital laboratories for those being followed in hospital consultations, and so the patient or caregiver was asked to bring the relevant INR laboratory results to the scheduled interview. Since our aim was to analyze TTR during the six months previous to the last INR measurement, the following data were required:
- (1)
the INR test result immediately prior to enrollment in the study;
- (2)
all INR test results during the six months prior to the last measurement; and
- (3)
the INR test result immediately prior to the above-mentioned six-month period.
Eleven patients were excluded as the data needed to calculate TTR were unavailable (patients had changed laboratory or had been unable to obtain all the results for the period under analysis). One case of recent AF with less than two INR measurements was excluded since TTR could not be calculated, as were two other patients who had discontinued their medication for more than seven days in order to undergo invasive procedures.
Thus, 74 (70%) of the 106 patients under classic OAC therapy were included in the analysis. Through consultation of medical records and personal interviews, data were collected on INR values and sociodemographic (age, gender, educational level and existence of a caregiver) and clinical variables (duration of AF and of OAC therapy) (Annex I), which were subsequently entered in a database constructed by the investigators using Microsoft Excel 2010®. TTR was calculated using the Rosendaal method to estimate the percentage of days within the therapeutic range, based on the laboratory results provided by the patient, using the Excel spreadsheet provided by INRPRO Healthcare System Solutions (http://www.inrpro.com/article.asp?id27).
The statistical analysis was performed using SPSS version 21.0® for Microsoft Windows®. To describe the population characteristics, measures of central tendency and dispersion (means and standard deviation or medians and 25th and 75th percentiles [P25 and P75]) were calculated for continuous variables, while absolute and relative frequencies were calculated for categorical variables. The relationship between TTR and clinical and sociodemographic variables was analyzed using Spearman's correlation and the Mann-Whitney U and Kruskal-Wallis tests. A level of significance of 0.05 was adopted.
In compliance with Portuguese law and the principles of confidentiality and privacy, written informed consent was obtained from all participants or their legal guardian. Participants’ anonymity was ensured by excluding any personal information enabling patients to be identified from the database. As this was a case series study carried out in the Fânzeres FHU, approval was obtained from the unit's supervisory team, who were responsible for monitoring the study, including its ethical integrity.
ResultsThe sociodemographic and clinical characteristics of the study population are shown in Table 1. The distribution of anticoagulation therapy duration was asymmetrical, with 16 patients (21.6%) anticoagulated for less than a year and others for up to 30 years.
Sociodemographic characteristics of the study population.
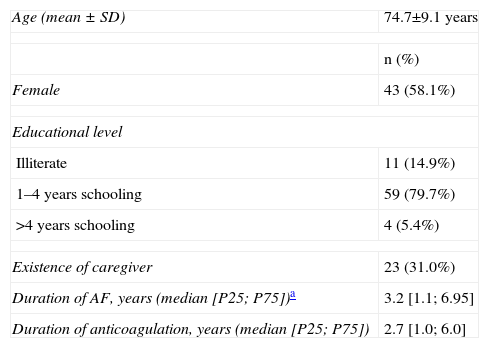
| Age (mean ± SD) | 74.7±9.1 years |
| n (%) | |
| Female | 43 (58.1%) |
| Educational level | |
| Illiterate | 11 (14.9%) |
| 1–4 years schooling | 59 (79.7%) |
| >4 years schooling | 4 (5.4%) |
| Existence of caregiver | 23 (31.0%) |
| Duration of AF, years (median [P25; P75])a | 3.2 [1.1; 6.95] |
| Duration of anticoagulation, years (median [P25; P75]) | 2.7 [1.0; 6.0] |
AF: atrial fibrillation; P25: 25th percentile; P75: 75th percentile; SD: standard deviation.
The results revealed a median TTR of 65.3% (Figure 2).
With regard to the relationship between TTR and the study variables, a positive and statistically significant association was found only with the duration of AF (Spearman's rho=0.477; p<0.001, r2=0.116) and of anticoagulation (Spearman's rho=0.5; p<0.001, r2=0.087). There was no statistically significant association with age (Spearman's rho=0.094; p=0.425), gender (p=0.08), educational level (p=0.763) or existence of a caregiver (p=0.636).
A high level of collinearity was detected between duration of AF and duration of anticoagulation (Spearman's rho=0.51; p<0.001, r2=0.647).
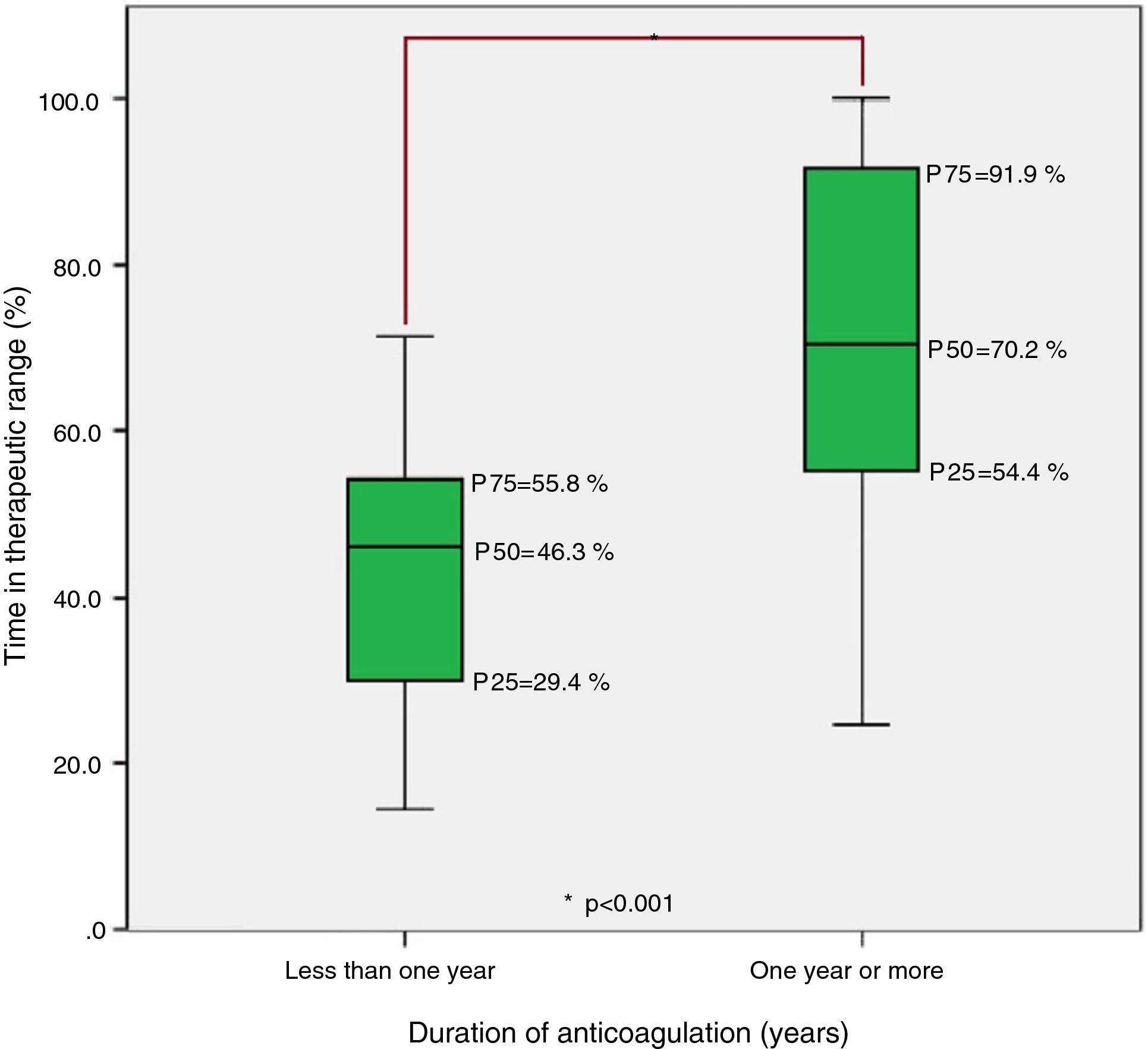
Finally, analysis of the group anticoagulated for less than one year and the group anticoagulated for one or more years revealed statistically significant differences in TTR, with median TTR significantly lower in the former (Figure 3).
DiscussionFânzeres FHU patients anticoagulated with classic OACs presented a median TTR of 65.3% (P25=48.3%; P75=86.8%), similar to the median value reported in the literature for southern European countries (64%) but below that of northern European countries (75%), which may be related to different methods of monitoring OAC therapy in these countries.8–17 The figure is slightly lower than the 70% proposed as good control by the European Society of Cardiology.7 The results point to an urgent clinical need for greater efforts within the community and families to improve patient adherence to therapy, as well as in monitoring possible drug and food interactions. For patients with good adherence but who still present poor control, replacing warfarin or acenocoumarol with one of the new OACs should be considered.
The study's strong point is that it is the first to characterize TTR in the everyday clinical practice of a Portuguese FHU in which monitoring of OAC therapy is carried out by laboratories in the community or in hospitals. However, given the study's observational nature, there are certain points to bear in mind concerning its limitations and the measures taken to minimize their effect. The issue of internal validity is discussed below.
Firstly, on the question of possible confounding factors, to which observational studies are particularly vulnerable, we believe there are biologically plausible mechanisms that can explain the moderate positive association found between TTR and duration of anticoagulation, since OAC therapy is begun at empirical doses, which are successively adjusted over a variable period of time until stable INR values and hence better anticoagulation control are achieved. In view of the high level of collinearity between the duration of AF and of OAC therapy, the association with duration of AF probably reflects its relationship with duration of anticoagulation, although there may be other factors contributing to this association.
The duration of AF and of anticoagulation explain only 11.6% and 8.7%, respectively, of the variation in TTR. The remainder may be due to other factors, particularly the accessibility of INR measurement and co-medication with drugs that interact with OACs, as reported in the literature.
However, the possibility of a type II error cannot be excluded: given the small number of participants, the study may have had insufficient power to detect differences between subgroups in the variables studied, such as educational level.
With regard to selection bias, although around 30% of the population were excluded from the analysis, of those excluded because of death, none died from thromboembolic or bleeding causes, and post-hoc analysis showed that the 11 individuals excluded due to insufficient INR data to calculate TTR did not differ significantly from those included in terms of sociodemographic or clinical characteristics. Furthermore, while cases were identified based on classification as ICPC-2 code K78 (Atrial fibrillation/flutter), we tried to minimize bias by widening the search to patients classified with other ICPC-2 codes that could have been used to classify this health problem – K79 (Paroxysmal tachycardia), K80 (Cardiac arrhythmia NOS), and K99 (Cardiovascular disease other).
Lastly, AF prevalence in Fânzeres FHU patients aged 40 or over was calculated at 1.73%, well below the 2.5% estimated for the Portuguese population aged 40 or over in the national reference study FAMA.1 Underdiagnosis could result in selection bias if it affects mainly patients with characteristics that are shown to be associated with worse or better TTR, but we have no data that would allow this hypothesis to be confirmed or refuted.
INR measurement is standardized between laboratories, so the TTR values in the study were not subject to measurement bias. However, we cannot exclude possible recall bias in some variables based on information provided by the patient or caregiver, particularly date of diagnosis and initiation of anticoagulation. We tried to minimize this problem by cross-referencing the information provided by patients with medical records, only using the former when the dates of diagnosis and initiation of OAC therapy were not clearly stated in the latter; however, information bias cannot be completely excluded due to errors in the records, especially since the records themselves may have been based on information provided by the patient.
A standard procedure was followed in the personal interviews and a standardized data collection form was used in order to reduce interviewer bias.
ConclusionsThe study revealed that TTR in the authors’ FHU is around 65%, which is lower than desirable. The duration of AF and of anticoagulation explains only a small part of the variation observed in TTR.
These results have clear clinical implications, pointing to the need for greater efforts in improving patients’ adherence to therapy, monitoring drug interactions and educating patients on food-drug interactions, as well as considering a change to one of the new OACs for patients with poor control despite good adherence to therapy.
Comparison of TTR in primary care units with and without their own laboratory facilities to monitor anticoagulation is also important in terms of health policies, since the possibility of including such facilities in the services provided by FHUs is currently under discussion.
Future studies will be required with larger populations to analyze other factors that may explain TTR variability, particularly access to INR measurement and different models for monitoring OAC therapy (in primary care units, by laboratories in the community or in hospital laboratories, or by patient self-monitoring using appropriate equipment). Other factors to be considered include individual patient characteristics affecting adherence to therapy, comorbidities, and possible drug interactions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pinho-Costa L, Moreira S, Azevedo C, et al. APOLO I: controlo da hipocoagulação na fibrilhação auricular. Rev Port Cardiol. 2015;34:337–345.



 OAC: oral anticoagulant;
OAC: oral anticoagulant; 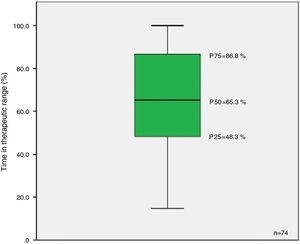 P25: 25th percentile;
P25: 25th percentile; 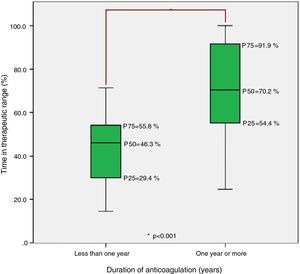 P25: 25th percentile;
P25: 25th percentile; 


