Pulmonary vein isolation (PVI) technique has become the cornerstone of atrial fibrillation (AF) catheter ablation. The objective of this study was to assess the efficacy and safety of extended antrum ablation based on electrophysiological substrate mapping plus PVI in AF patients who underwent cryoballoon ablation.
MethodsIn this observational study, a total of 121 paroxysmal AF patients and 80 persistent AF patients who did not achieve the procedure endpoint after cryoballoon ablation received extra extended antrum ablation (EAA) based on electrophysiological substrate mapping via radiofrequency ablation (EAA group). As a control group (PVI group), among paroxysmal AF and persistent AF patients, we conducted a propensity score-matched cohort, in whom only PVI was completed.
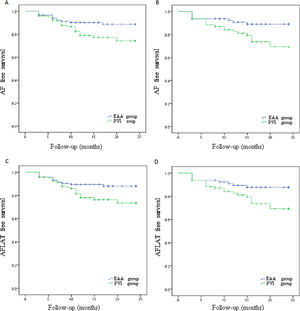
ResultsThe average follow-up time was 15.27±7.34 months. Compared with PVI group, paroxysmal AF patients in the EAA group had a significantly higher rate of AF-free survival (90.1% vs. 80.2%, p=0.027) and AF, atrial flutter, or atrial tachycardia (AFLAT) -free rate survival (89.3% vs. 79.3%, p=0.031). Persistent AF patients in the EAA group also had a significantly higher rate of AF-free survival (90.0% vs. 75.0%, p=0.016) and AFLAT-free survival (88.8% vs. 75.0%, p=0.029) than PVI group. Complication rates did not significantly differ between both groups, in either paroxysmal AF or persistent AF patients.
ConclusionOur findings demonstrate that extra extended antrum ablation based on electrophysiological substrate mapping is effective and safe. Moreover, the strategy can improve the outcome of AF cryoablation.
A técnica de isolamento das veias pulmonares (IVP) tornou-se a pedra angular da ablação por cateter da fibrilhação auricular (FA). O objetivo deste estudo consistiu em avaliar a eficácia e a segurança da ablação antral alargada com base no mapeamento do substrato eletrofisiológico, para lá do IVP em doentes com FA, submetidos a crioablação.
MétodosNeste estudo observacional, 121 doentes com FA paroxística e 80 doentes com FA persistente que não alcançaram o objetivo do procedimento após a crioablação foram submetidos a ablação antral alargada com base no mapeamento do substrato eletrofisiológico, através de ablação por radiofrequência (grupo de Ablação Antral Alargada, grupo AAA). Como grupo controlo (grupo do Isolamento das Veias Pulmonares, grupo IVP), em doentes com FA paroxística e com FA persistente, realizámos um score de propensão agrupado, no qual só foi efetuado o IVP.
ResultadosO tempo médio de seguimento foi 15,27±7,34 meses. Quando comparados com o grupo IVP, os doentes com FA paroxística no grupo AAA apresentaram uma taxa significativamente mais elevada de sobrevivência livre de FA (90,1% versus 80,2%, p=0,027) e de sobrevivência livre de AFLAT (FA, flutter auricular ou taquicardia auricular) (89,3% versus 79,3%, p=0,031). Os doentes com FA persistente no grupo AAA também apresentaram uma taxa significativamente mais elevada de sobrevivência livre de FA (90,0% versus 75,0%, p=0,016) e de sobrevivência livre de AFLAT (88,8% versus 75,0%, p=0,29) do que no grupo de IVP. As taxas de complicações não diferiram significativamente entre dois grupos, tanto nos doentes com FA paroxística como nos com FA persistente.
ConclusãoOs nossos resultados demonstraram que a ablação antral alargada com base no mapeamento de substrato eletrofisiológico é eficaz e segura. Além disso, a estratégia pode melhorar os resultados da FA por crioablação.
Pulmonary vein isolation (PVI) is the cornerstone of atrial fibrillation (AF) catheter ablation.1 Cryoballoon ablation (CBA) is a new treatment for AF, with the advantage of creating a contiguous and transmural lesion around each pulmonary vein in a relatively simple manner.2 Several studies2–5 have shown comparable clinical outcomes between traditional radiofrequency ablation (RFA) and CBA. However, considering the elusive mechanism in the progression of AF, the PVI-only strategy has not always guaranteed long-term maintenance of sinus rhythm regardless of the classification of AF. Atrial substrate remodeling is one of the important mechanism underlying the occurrence and persistence of AF.6 Several empirical extra pulmonary vein (PV) ablation strategies for substrate modification have been applied to AF patients,7–11 and have been found to be beneficial in several trials.10,11 Nevertheless, outcomes, including non-recurrence rate and complication rate, were inconsistent among strategies.7–9 This may due to the reconnections of PVI and linear ablation, as well as the possibility of normal substrate lesions, which lead to ablation-related atrial tachycardia. Therefore, a more individualized, efficient, and effective ablation strategy is required without unnecessary cardiac tissue damage.
Our center implemented an extended antrum ablation strategy targeting patients who had difficulties in achieving the procedure endpoint after CBA (procedure endpoint is defined in the “Operation Strategy and Intraoperative Record” section). The extended antrum ablation strategy was defined as follow: Patients in this group received electrophysiological substrate mapping at a distance of >5 mm from the ostia of the pulmonary veins (the antrum of pulmonary veins) to discover the target substrate, a near field potential of 0.5 mV and voltage area higher than 0.5 mV. With the guidance of substrate mapping, we applied RFA to target substrate to complete extended antrum ablation. Our study was designed with the objective of assessing the efficacy and safety of extended antrum ablation strategy besides PVI in paroxysmal AF and persistent AF patients. We present the following article in accordance with the CONSORT reporting guidelines.
Materials and methodsResearch subjectsIn this observational study, from May 2014 to August 2018, a total of 121 consecutive paroxysmal AF patients and 80 consecutive persistent AF patients received extra extended antrum ablation based on electrophysiological substrate mapping (extended antrum ablation group (EAA)). Propensity score-matched cohorts were created based on variables which were expected to be potential confounders associated with AF ablation, including gender, age, classification of AF, CHA2DS2-VASc score, left atrial diameter (LAD) and left ventricular ejection fraction (LVEF). Patients were matched with an equal proportion of paroxysmal AF and persistent AF patients who received only PVI using cryoballoon in the same period (PVI group). The PVI group was the control, and EAA group was the comparator. All patients met AF diagnostic standards,12 and did not have any history of AF catheter ablation.
Preoperative preparationAfter hospitalization, all patients underwent CHA2DS2-VASc score evaluations for cerebral arterial thrombosis. Before being operated, all patients underwent transthoracic echocardiography to measure indexes such as LAD and LVEF. Transesophageal echocardiography was then administered to exclude left atrial thrombus and left atrial and pulmonary venous computed tomography angiography (CTA) was performed to assess the left atrium size and PV anatomical structure. All patients signed informed consent for the operation after a detailed preoperative conversation.
Operation strategy and intraoperative recordAll procedures were performed under topical anesthesia by two experienced operators. During the procedure, the left and right femoral veins were punctured, and a decapolar catheter was placed to reach the coronary sinus through the left femoral vein. A quadrupole catheter was also placed at the superior vena cava through the left femoral vein for pacemaking detection of phrenic nerve injuries. The atrial septum was punctured followed by heparinizing according to weight, and an 8F 65-cm sheathing canal (SL1, Abbot, USA) was sent to the left atrium. Left atrial and PV radiographs were performed to mark the PV ostia size and position. The FlexCath sheath (CryoCath, Medtronic, USA) was exchanged to send the Achieve Mapping Catheter with the cryoballoon (28-mm Arctic Front or Arctic Front Advance, Medtronic, USA). Using the EnSite NavX 3D-mapping system, the cryoballoon and catheter system were positioned at the ostia and antrum of the left superior pulmonary vein (LSPV). The cryoballoon was inflated and contact and selective PV radiography was performed to ensure complete occlusion. Liquid nitrogen was then introduced to perform the cryoablation to isolate the LSPV. The same steps were repeated to isolate the left inferior pulmonary vein (LIPV), right superior pulmonary vein (RSPV), and right inferior pulmonary vein (RIPV), thereby achieving bidirectional isolation. PV radiography was also used to detect right middle pulmonary vein (RMPV) or left common pulmonary vein (LCPV) during the procedure. During the right-side PV cryoablation, continuous pacing in the superior vena cava paced the phrenic nerve so that the cryoablation could be immediately halted once the diaphragm contraction began to weaken. Cryoablation time was 120-240 s.
After achieving immediate PVI using the abovementioned CBA procedure, the endpoint was defined as follow: Paroxysmal AF patients underwent burst pacing (frequency 200-300 ms) without inducing AF immediately and at least 30 minutes after ablation. In persistent AF patients, AF did not occur after receiving synchronized electrical cardioversion, and the observation continued at least 30 minutes after ablation.
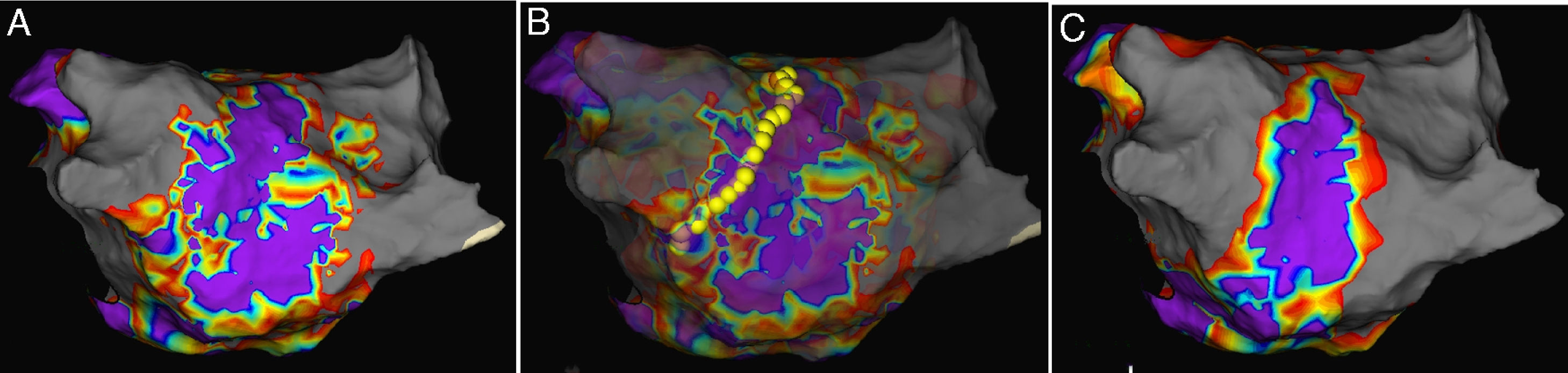
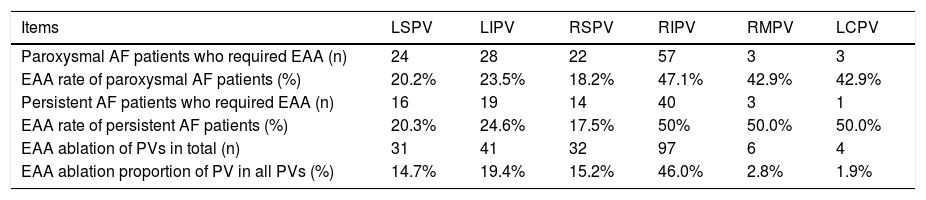
If the procedure endpoint was not achieved, electrophysiological substrate mapping was applied to discover the target substrate in the pulmonary vein atrum, a distance of >5 mm from the ostia of the PVs (a representative example is shown in Figure 1A). All the substrate mapping was performed during sinus rhythm. A near field potential of 0.5 mV and voltage area higher than 0.5 mV were considered as the target substrate.13 With the guidance of substrate mapping, a cool saline irrigation catheter (Therapy Cool Path Duo, Abbot, USA) was implanted to complete RFA procedure (a representative example is shown in Figure 1B). After ablation, left atrium-PV antrum substrate mapping was performed again to ensure the elimination of any potential that might lead to AF (a representative example is shown in Figure 1C). For patients with other arrhythmias, such as supraventricular tachycardia or atrial flutter, RFA was simultaneously applied for the treatment. During the procedure, characteristics, including temperature for 30s at the beginning of ablation, nadir temperature, freeze cycles, cryoballon isolation time, cryoablation application time, extended antrum ablation or not, and position of extended antrum ablation were recorded. After the procedure, total cryoballon isolation time, total freeze cycles, X-ray exposure time and quantity, as well as complications such as phrenic nerve injuries, pericardial tamponade, arteriovenous fistula and hematoma, were recorded.
Representative examples of antrum ablation strategy. A. Electrophysiological substrate mapping showed target substrate according to EnSite NavX system; B. Radiofrequency ablation based on substrate mapping on Left pulmonary vein; C. Postablation substrate mapping to ensure the elimination of target substrate.
After the procedure, patients took oral anticoagulant drugs for at least three months. After three months and the evaluation of stroke risk, a decision was made on whether to continue the drugs. Within three months of the operation, patients took oral antiarrhythmic drugs depending on their need for rate control. Three, six, nine and twelve months after operation, all patients underwent a 72h Holter exam. One year after the operation, patients took the Holter re-examination every six months. During this period, if patients had arrhythmic symptom, a normal electrocardiogram (ECG) or Holter would be recommended for follow-up. Postoperation recurrence, which was also the endpoint of this study, was defined as 30s longer recurrence of AFLAT detected via normal ECG or Holter after a three month blanking period.
Statistical analysisSPSS 22.0 software was used for the statistical analysis. Patients in PVI group were matched 1:1 to patients in EAA group based on the estimated propensity score from a logistic regression model. The model included gender, age, classification of AF, CHA2DS2-VASc score, left atrial diameter and LVEF as covariates. Analyses were conducted in the matched cohorts. Measurements were presented as the mean±SD (x¯±s). Numerical data were presented as a number and percentage. Baseline characteristics, intraoperative data and key follow-up outcomes were compared between PVI group and EAA group with the use of the chi-square test, Fisher's exact test and two-sample t-test. The AFLAT-free rate and AF-free rate were analyzed using a Kaplan-Meier survival curve, and log-rank tests were applied for comparison. A p-value of less than 0.05 was considered to indicate statistical significance.
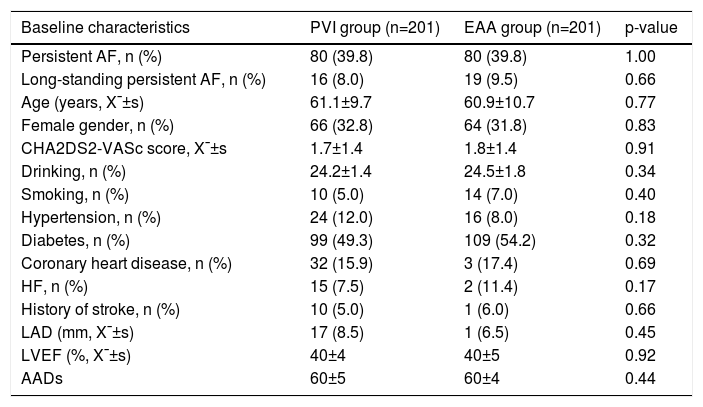
ResultsBaseline dataThe study cohort included 121 paroxysmal AF patients and 80 persistent AF patients who received extra extended antrum ablation based on electrophysiological substrate mapping. As a control group, we conducted a 1:1 propensity score-matched cohort, in whom only PVI was completed. Finally, a total of 242 paroxysmal AF patients (n=121 in PVI group and n=121 in EAA group) and a total of 160 persistent AF patients (n=80 (54.3% persistent AF, 45.7% long-standing persistent AF) in PVI group and n=80 (45.7% persistent AF, 54.3% long-standing persistent AF) in EAA group were enrolled in our study. The baseline characteristics of AF patients in PVI group and EAA group are shown in Table 1. After the propensity score was matched, the baseline characteristics of the two groups did not present significant difference (p>0.05).
Baseline clinical characteristics of patients with atrial fibrillation (n=402).
| Baseline characteristics | PVI group (n=201) | EAA group (n=201) | p-value |
|---|---|---|---|
| Persistent AF, n (%) | 80 (39.8) | 80 (39.8) | 1.00 |
| Long-standing persistent AF, n (%) | 16 (8.0) | 19 (9.5) | 0.66 |
| Age (years, X¯±s) | 61.1±9.7 | 60.9±10.7 | 0.77 |
| Female gender, n (%) | 66 (32.8) | 64 (31.8) | 0.83 |
| CHA2DS2-VASc score, X¯±s | 1.7±1.4 | 1.8±1.4 | 0.91 |
| Drinking, n (%) | 24.2±1.4 | 24.5±1.8 | 0.34 |
| Smoking, n (%) | 10 (5.0) | 14 (7.0) | 0.40 |
| Hypertension, n (%) | 24 (12.0) | 16 (8.0) | 0.18 |
| Diabetes, n (%) | 99 (49.3) | 109 (54.2) | 0.32 |
| Coronary heart disease, n (%) | 32 (15.9) | 3 (17.4) | 0.69 |
| HF, n (%) | 15 (7.5) | 2 (11.4) | 0.17 |
| History of stroke, n (%) | 10 (5.0) | 1 (6.0) | 0.66 |
| LAD (mm, X¯±s) | 17 (8.5) | 1 (6.5) | 0.45 |
| LVEF (%, X¯±s) | 40±4 | 40±5 | 0.92 |
| AADs | 60±5 | 60±4 | 0.44 |
AADs: antiarrhythmic drugs; AF: atrial fibrillation; EAA: extended antrum ablation; HF: heart failure; LAD: left atrial diameter; LVEF: left ventricular ejection fraction; PVI: pulmonary vein isolation.
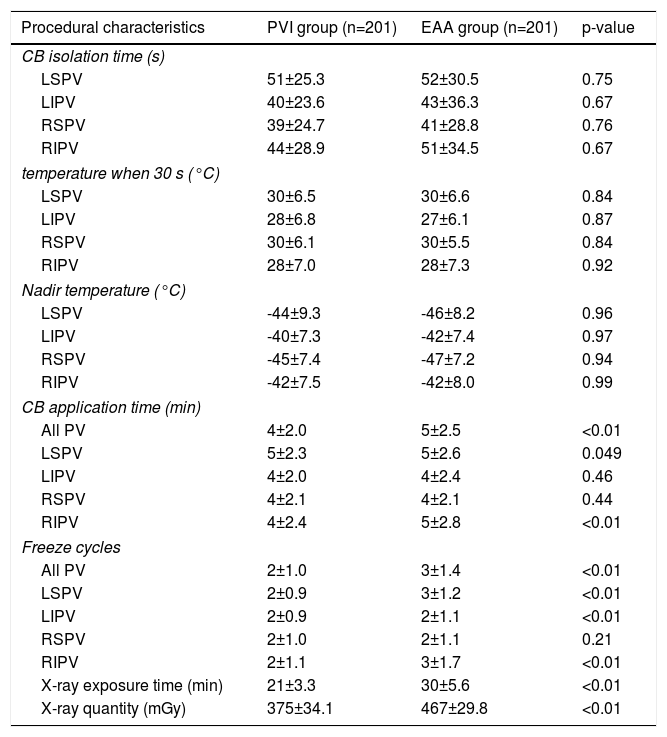
In PVI and EAA group, all patients achieved 100% PVI. Table 2 shows the intraoperative data for paroxysmal AF patients and persistent AF patients in two groups. Except for the RSPV, the freeze cycles for each PV in the EAA group were higher than those in the PVI group. Cryoablation times for the LSPV and RIPV were significantly higher in the EAA group than in the PVI group. X-ray exposure time was significantly longer, and X-ray exposure quantity was significantly higher in the EAA group than in the PVI group (p<0.05).
Procedural characteristic between the pulmonary vein isolation and extended antrum ablation groups in atrial fibrillation patients.
| Procedural characteristics | PVI group (n=201) | EAA group (n=201) | p-value |
|---|---|---|---|
| CB isolation time (s) | |||
| LSPV | 51±25.3 | 52±30.5 | 0.75 |
| LIPV | 40±23.6 | 43±36.3 | 0.67 |
| RSPV | 39±24.7 | 41±28.8 | 0.76 |
| RIPV | 44±28.9 | 51±34.5 | 0.67 |
| temperature when 30 s (°C) | |||
| LSPV | 30±6.5 | 30±6.6 | 0.84 |
| LIPV | 28±6.8 | 27±6.1 | 0.87 |
| RSPV | 30±6.1 | 30±5.5 | 0.84 |
| RIPV | 28±7.0 | 28±7.3 | 0.92 |
| Nadir temperature (°C) | |||
| LSPV | -44±9.3 | -46±8.2 | 0.96 |
| LIPV | -40±7.3 | -42±7.4 | 0.97 |
| RSPV | -45±7.4 | -47±7.2 | 0.94 |
| RIPV | -42±7.5 | -42±8.0 | 0.99 |
| CB application time (min) | |||
| All PV | 4±2.0 | 5±2.5 | <0.01 |
| LSPV | 5±2.3 | 5±2.6 | 0.049 |
| LIPV | 4±2.0 | 4±2.4 | 0.46 |
| RSPV | 4±2.1 | 4±2.1 | 0.44 |
| RIPV | 4±2.4 | 5±2.8 | <0.01 |
| Freeze cycles | |||
| All PV | 2±1.0 | 3±1.4 | <0.01 |
| LSPV | 2±0.9 | 3±1.2 | <0.01 |
| LIPV | 2±0.9 | 2±1.1 | <0.01 |
| RSPV | 2±1.0 | 2±1.1 | 0.21 |
| RIPV | 2±1.1 | 3±1.7 | <0.01 |
| X-ray exposure time (min) | 21±3.3 | 30±5.6 | <0.01 |
| X-ray quantity (mGy) | 375±34.1 | 467±29.8 | <0.01 |
CB: cryoablation; EAA: extended antrum ablation; LIPV: left inferior pulmonary vein; LSPV: left superior pulmonary vein; PVI: pulmonary vein isolation; RIPV: right inferior pulmonary vein; RSPV: right superior pulmonary vein.
Table 3 shows the position characteristics of extended antrum ablation based on electrophysiological substrate mapping in both paroxysmal AF patients and persistent AF patients in EAA group. We defined the extended antrum ablation rate (EAA rate) as proportion of PV that required adjacent extended antrum ablation. RMPVs and LCPVs were detected by PV radiography during the procedure. Table 3 shows that EAA rates of RIPV, RMPV and LCPV in both paroxysmal AF and persistent AF patients were significantly higher when compared with that of LSPV, LIPV and RSPV (p<0.01). In all PVs that required adjacent extended antrum ablation, area adjacent to RIPV needed significantly more extended antrum ablation than other PVs (p<0.01).
Adjacent extended antrum ablation of each PVs in EAA group.
| Items | LSPV | LIPV | RSPV | RIPV | RMPV | LCPV |
|---|---|---|---|---|---|---|
| Paroxysmal AF patients who required EAA (n) | 24 | 28 | 22 | 57 | 3 | 3 |
| EAA rate of paroxysmal AF patients (%) | 20.2% | 23.5% | 18.2% | 47.1% | 42.9% | 42.9% |
| Persistent AF patients who required EAA (n) | 16 | 19 | 14 | 40 | 3 | 1 |
| EAA rate of persistent AF patients (%) | 20.3% | 24.6% | 17.5% | 50% | 50.0% | 50.0% |
| EAA ablation of PVs in total (n) | 31 | 41 | 32 | 97 | 6 | 4 |
| EAA ablation proportion of PV in all PVs (%) | 14.7% | 19.4% | 15.2% | 46.0% | 2.8% | 1.9% |
AF: atrial fibrillation; EAA: extended antrum ablation; LCPV: left common pulmonary vein; LIPV: left inferior pulmonary vein; LSPV: left superior pulmonary vein; PV: pulmonary vein; RIPV: right inferior pulmonary vein; RMPV: right middle pulmonary vein; RSPV: right superior pulmonary vein.
The average follow-up time of paroxysmal AF patients and persistent AF patients was 15.27±7.34 months. During the follow-up, we found 67 cases of recurrence, including 64 cases of recurrent AF and three cases of recurrent atrial flutter or atrial tachycardia (two cases in EAA group and one in the PVI group), with no significant difference in the recurrent atrial arrhythmia rate (p=0.56). Among 67 cases of recurrence, 38 of them are paroxysmal AF patients (25 in PVI group and 13 in EAA group), 29 of them are persistent AF patients (20 in PVI group and 9 in EAA group).
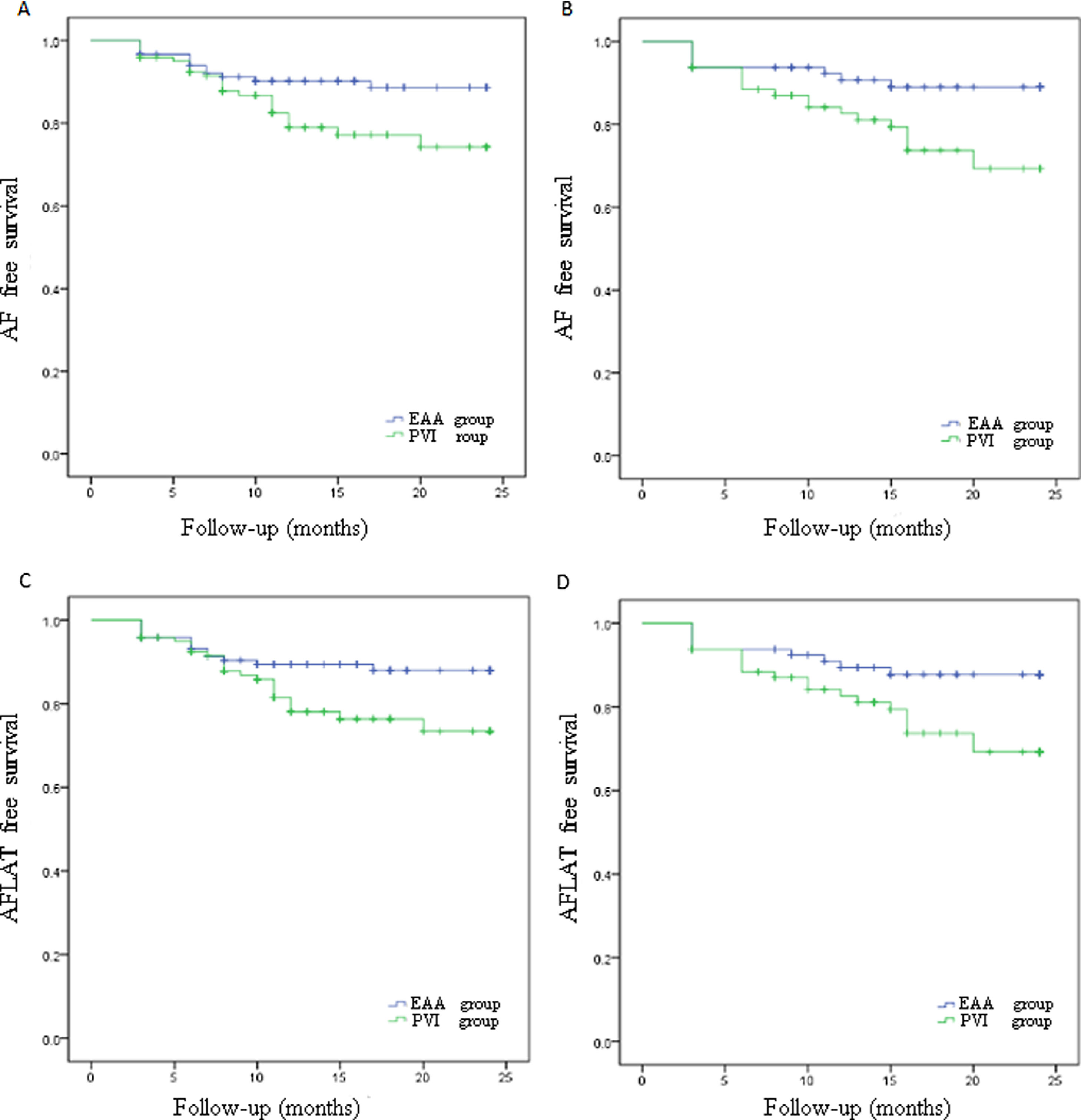
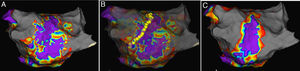
Paroxysmal AF patients in PVI group had an AF-free rate of 80.2%, which was significantly lower (p=0.027; Figure 2A) than the 90.1% in the EAA group. Paroxysmal AF patients in the PVI group had an AFLAT-free rate of 79.3%, which was significantly lower (p=0.031; Figure 2B) than the 89.3% in the EAA group. Persistent AF patients in the PVI group had an AF-free rate of 75.0%, which was statistically significantly lower than the 90.0% in the EAA group (p=0.016; Figure 2C). Persistent AF patients in the PVI group had an AFLAT-free rate of 75.0%, which was statistically significantly lower than the 88.8% in the EAA group (p=0.029; Figure 2D).
Kaplan-Meier curve showing the atrial fibrillation, atrial flutter, or atrial tachycardia-free rate of paroxysmal atrial fibrillation patients and persistent atrial fibrillation patients.
(A) AF-free survival rate of paroxysmal AF patients; (B) AF-free survival rate of persistent AF patients; (C) AFLAT-free survival rate of paroxysmal AF patients; (D) AFLAT-free survival rate of persistent AF patients.
AF: atrial fibrillation; AFLAT: atrial fibrillation, atrial flutter, or atrial tachycardia.
Complications during cryoablation were as follows: a total of three in 201 patients in the PVI group experienced complications, and the occurrence rate was 1.5%. A total of five in 201 patients in EAA group experienced complications, and the occurrence rate was 2.5%. The difference was not statistically significant between the two groups (p=0.47). One patient in the EAA group who underwent LSPV adjacent extended antrum ablation experienced postoperative LSPV stenosis. No patients in the PVI group experienced PV stenosis. Three patients experienced phrenic nerve injuries/paralysis (one case in EAA group and two cases in the PVI group, all of which occurred at the time of RSPV cryoablation). One patient recovered before the end of the operation, and one patient recovered three months after the operation. No significant difference was observed in the occurrence rate of phrenic nerve injuries/paralysis between the two groups (p=0.559). Other complications included one case of hemopneumothorax and one case of false aneurysm among patients with combined CB and RF ablation, and one case of arterial aneurysm and one case of atrial septal defects among patients in PVI group.
DiscussionThe principal finding of our study was that extended antrum ablation strategy can improve mid-term to long-term AFLAT-free rate of CBA without increasing the risks of complications both in paroxysmal AF and persistent AF patients. We chose patients who did not achieve the procedural endpoint after PVI to undergo electrophysiological substrate mapping to discover the target substrate in the pulmonary vein antrum. Thus, we conducted RFA based on substrate mapping beyond PVI, which was the innovative strategy of our center.
Extended antrum ablation strategy based on substrate mappingOur center reviewed several pivotal studies on procedural endpoints and recurrence rates.14–16 We combined the rationale of excitation due to sympathomimetic effects such as electrical stimulation or drug stimulation to induce AF17 and set our procedure endpoint. The procedure endpoint was assessed on condition that patients achieved PVI with no potential recovery transmission detected after ablation immediately or at least 30 minutes later. The procedural endpoint was defined as follow: Paroxysmal AF patients and persistent AF patients after receiving synchronized electrical cardioversion underwent burst electrical stimulation without inducing AF.
Major global centers18–21 have adopted first-generation 23-mm cryoballoon or RFA to assist isolation for touch-up ablation for patients who did not reach their procedural endpoint. However, prolonging the cryoablation time may lead to increasing risks of complications such as phrenic nerve injuries and left atrial fistula,22,23 which could result in a higher phrenic nerve injury rate of second-generation cryoballoons.24 In consideration of the above, our center adopted RFA using cool saline irrigation catheter for assisting ablation.
Therefore, once the procedure endpoint was not achieved after PVI verification, we adopted the left atrium-PV antrum substrate mapping using an Achieve Mapping Catheter with the guidance of the EnSite NavX 3D-mapping system. All the substrate mapping was performed during sinus rhythm. Electrophysiological substrate mapping was applied to discover target substrate in the pulmonary vein antrum. A near field potential of 0.5 mV and voltage area higher than 0.5 mV were considered as the target substrate. Based on substrate mapping, Therapy Cool Path Duo was applied for necessary pulmonary vein gaps ablation and subsequent extended antrum ablation. After the ablation, we applied the left atrium-PV antrum substrate mapping secondly to ensure the elimination of any possible potential that might lead to AF. To our knowledge, extended antrum ablation strategy based on electrophysiological substrate mapping is an innovative strategy, the benefit of which will be discussed in the following section of this article.
Clinical data analysis of patients with extended antrum ablation strategyCryoballoon ablation, as one of the most types of AF catheter ablation, has the advantage of creating an efficient PVI compared to traditional radiofrequency ablation. Increased freezing time after achieving immediate PVI with cryoballoon did not improve the AFLAT-free rate of AF patients,25–27 which indicates that cryoablation can quickly create contiguous and transmural lesion around the tissue with which it is in contact. For those patients who did not achieve the procedural endpoint while conducting electrophysiological substrate mapping, our experience indicated that target substrate in left atrial-PV antrum found during electrophysiological substrate mapping required extra extended antrum ablation.
An additional RMPV together with LCPV are the two most common PV pattern variations.28 Although cryoballoon can isolate a certain proportion of RMPV and LCPV regardless of anatomic variation,29,30 there was still significantly more area adjacent to RMPV and LCPV that required extended antrum ablation among patients who did not achieve the procedure endpoint.
Previous findings showed that patients with RMPVs tend to have a higher frequency of atrial arrhythmias including AF than those with normal four PVs pattern,31 which seems to indicate that RMPV is more likely to be a trigger of AF. Most RMPVs have a smaller diameter than major PVs, while the cross-sectional area may be comparable to major PV dimensions.32 Due to the contrast in size of RMPV ostia and cross-sectional area, even if the cryoballoon reached the contiguous lesion around RMPV ostia, there may still be some potential adjacent to the antrum that relates to AF occurrence. If there is an LCPV, the antrum from which the PV flows into the left atrium will be larger than normal. Proietti et al.4 found that an electrophysiological substrate maintains the AF at the left atrium-PV antrum, and this substrate is richer when the LCPV cross-sectional dimension is larger. Thus, the existence of LCPV requires more adjacent extended antrum ablation to achieve the procedural endpoint.
Among all PVs, the area adjacent to RIPV required the most extended antrum ablation. Compared with RSPV, LIPV and LSPV, the anatomical position of RIPV ostia is relatively lower and flatter. If the position of the atrial septal puncture is high, it may be more difficult to achieve perfect RIPV contact, which leaves behind possible antrum potential.
Clinical outcome analysisAll 402 patients in our cohort were effectively followed up in this research. The AFLAT-free rate for patients with paroxysmal AF and persistent AF who underwent PVI alone via CBA were 80.2% and 75.0%. The AF-free rate for patients with paroxysmal AF and persistent AF who underwent PVI alone via CBA was 79.3% and 75.0%. Our center has comparable mid-term to long-term outcome of AFLAT-free rate with those of other major centers using CBA or RFA for PVI33–36 Randomized data using CBA for PVI have demonstrated similar outcomes to RFA not only in paroxysmal AF patients but also in persistent AF and long-standing persistent AF patients.37 To our knowledge, there are no acknowledged extra-PV ablation strategies proven to be inferior to PVI-only strategy due to inconsistent evidence.7–11 Our study selected patients who did not achieve the procedural endpoint after PVI to explore the possible relationship between pathological antrum electrophysiological substrate of PV and clinical outcome improvement, especially in patients with persistent AF and long-standing persistent AF.
Several clinical studies20,21,35,38 have suggested that RFA and CBA do not have significantly different outcomes among patients with PV-recovered transmission and without PV-recovered transmission after complete isolation of natural and latent recovered potential. Therefore, ablation merely focusing on PVI strategy is not always sufficient for maintaining sinus rhythm after AF catheter ablation. Electrophysiological substrates can easily maintain AF,6 and high-frequency re-entry wavelets can maintain AF at the PV antrum, while extended antrum ablation strategy can modify the atrial substrate and eliminate re-entry wavelets. Due to the same embryological origin between left posterior atrium and PVs, the left posterior atrium related to the AF maintenance mechanism can also be partially eliminated39 via extended antrum ablation based on substrate mapping. In addition, one important mechanism of persistent AF maintenance lies in the driving of the left posterior atrium,11 which can explain the reason why extended antrum ablation strategy could significantly benefit patients with persistent AF. Apart from this, a highly activated left atrial automatic nervous system serves as an important physiopathological mechanism for inducing AF.40 Verma et al.41 reported that ablating the PV antrum could eliminate partial automatic nerve clusters. Those four factors together account for the benefit of the extended antrum ablation strategy.
The use of cryoballoons has the advantage of creating a contiguous lesion around each pulmonary vein in a relatively simple and efficient manner, which leads to complete PVI in regular PVs under most circumstance. However, when it comes to extended antrum ablation, we admit that cryoballoon has the disadvantage of not being as versatile as an RF catheter and it did not have a matched mapping system.
In summary, extra extended antrum ablation based on electrophysiological substrate mapping can increase the AF-free rate by eliminating the partial triggering and maintenance mechanisms of AF. Moreover, this method improved the clinical outcomes without increasing the risks of ablation-related atrial arrhythmia such as atrial flutter and atrial tachycardia. Ablation under the guidance of accurate electrophysiological substrate mapping rather than empirical substrate modification is individualized and effective without unnecessary cardiac tissue damage. The limited cryoablation times of each PV are efficient and safe, which did not result in a higher phrenic nerve injury rate. In terms of other complications, except for one patient who suffered from PV stenosis in the EAA group, extended antrum ablation strategy did not increase the occurrence of AF complications in cryoablation patients. Therefore, extra extended antrum ablation based on electrophysiological substrate mapping via RFA following CBA on PVI is an optimal strategy of efficient and individual for AF catheter ablation.
LimitationsSeveral limitations should be considered in interpreting our study. First, this observational study analyzed the follow-up results of patients who underwent monocenter operations performed by more than one operator. Therefore, our evidence may lack universal clinical implications compared with prospective multicenter randomized controlled trials. Second, the number of long-standing persistent AF patients was limited in our study cohort, which also limits the extrapolation of extended antrum ablation strategy in the whole range of non-paroxysmal AF patients. Third, using not originally matched achieve catheters in the mapping system instead of current technology of greater value was also a limitation in our study. Fourth, combining a mapping system and RF ablation catheter truly resulted in a more costly procedure. Besides, considering economic and medical ethical factors, difficulties existed in applying electrophysiological substrate mapping to every patient after procedure. Due to this reason, our study merely applied extended antrum ablation strategy to patients who did not achieve the procedure endpoint. Hence, a randomized trial with a larger cohort could be considered to confirm the benefit of extended antrum ablation strategy.
ConclusionsExtra extended antrum ablation based on electrophysiological substrate mapping is effective and safe. Moreover, the strategy can improve the outcome of AF cryoablation.
DeclarationThis study has been performed with the approval of Ethics Committee of Fujian Provincial Hospital and with appropriate participant informed consent in compliance with the Declaration of Helsinki. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author contributionsAdministrative support: Zhang JC, Huang QL and Lin YZ; Provision of study materials or patients: Chen L, Chen JQ and Zou T; Collection and assembly of data: Lian LL, Yang ZP, Wu MQ and Zou T; Data analysis and interpretation: Liao XW and Lin W; Manuscript writing: All authors; Final approval of manuscript: All authors.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful for the support from the Key Clinical Specialty Discipline Construction Program of Fujian. This work was supported by Natural Science Foundation of Fujian Province [Nos: 2015J01370, 2019J01189], Key Innovation Project of Fujian Province Health Department [Nos: 2017-CX-6], the High-level Hospital Grants from Fujian Provincial Hospital [2017LHJJ02] and the Leading Key Projects of Science and Technology Plan in Fujian Province [Nos: 2018Y0014].