In patients with cryptogenic stroke, one of the most frequently found abnormalities is patent foramen ovale (PFO). Percutaneous ‘deviceless’ systems based on surgical suture-mediated PFO closure have recently been introduced and show a favorable efficacy and safety profile with clear advantages.
ObjectivesTo present procedural details of the technique and baseline characteristics of patients who underwent the procedure in our center.
MethodsA single-center prospective observational registry was established between February 2020 and February 2021, to assess the safety, efficacy and possible advantages of a novel percutaneous PFO closure system (NobleStitch® EL). Patient and PFO characteristics as well as technical features were collected for analysis.
ResultsTwenty-three patients were considered suitable for this technique after transesophageal echocardiography. Their mean age was 51 years and 69.5% were women. Most patients (91.3%) had a history of cryptogenic stroke. PFO closure with the NobleStitch® system was successfully performed in all patients. All procedures were performed under local anesthesia and fluoroscopic monitoring. The mean duration of the procedure was 52 min and median contrast dose used was 187 ml. Median radiation dose absorbed per patient was 61.5 Gy cm2. All patients were discharged asymptomatic 24 hours after the procedure with no peri- or postprocedural complications recorded.
ConclusionSuture-mediated PFO closure represents a valid and safe alternative to traditional umbrella-like devices, and is feasible in the majority of PFO anatomies. Follow-up information, results of larger series and clinical trials may possibly validate this technique as the first choice for PFO closure.
Em doentes com acidente vascular cerebral (AVC) criptogénico, uma das alterações mais frequentes é a presença de um foramen oval patente (FOP). A técnica percutânea de sutura baseada no encerramento de FOP através de sutura cirúrgica foi recentemente introduzida, mostrando eficácia e perfil de segurança vantajosos.
ObjetivosApresentar os detalhes desta técnica e documentar as características dos doentes do nosso centro.
MétodosRegisto observacional prospetivo unicêntrico realizado entre fevereiro de 2020 e fevereiro de 2021, para avaliar segurança, eficiência e vantagens do novo sistema de encerramento de FOP (NobleStitch® EL). As características dos doentes e da técnica foram recolhidas para análise.
ResultadosApós análise do ETE, 23 doentes foram considerados adequados para esta técnica. A idade média dos doentes foi 51 anos e 69,5% eram mulheres. A maioria dos doentes (91,3%) tinha história de AVC criptogénico. O encerramento de FOP com o sistema NobleStitch® foi realizado com sucesso em todos os doentes. Os procedimentos foram realizados com anestesia local e monitoração fluoroscópica. A duração média do procedimento foi 52 minutos e a mediana do contraste utilizado foi 187 ml. A dose média de radiação absorvida por doente foi 61,5 Gy cm2. Todos os doentes receberam alta assintomáticos 24 horas após o procedimento. Nenhuma complicação peri ou pós-procedimento foi registada.
ConclusãoO encerramento de FOP por sutura representa uma alternativa válida e segura aos dispositivos tradicionais, viável na maioria das anatomias. Dados sobre seguimento, resultados de estudos maiores e ensaios clínicos poderão validar esta técnica como primeira escolha para o encerramento de FOP.
Stroke is the leading cause of death in Portugal, accounting for 9.9% of mortality nationwide (11235 deaths) in 2018, according to the latest available data from Statistics Portugal (INE). In 2018, an estimated 11388 potential years of life were lost due to cerebrovascular disease.1
In patients with cryptogenic stroke, one of the most frequently found abnormalities in the complementary investigation is patent foramen ovale (PFO).2 In the adult population, the prevalence of PFO is 20-25% and in patients who suffer a cryptogenic stroke, 40-50% have this anatomic variant.3
Right heart catheterization demonstrating a guidewire crossing the septum is the most accurate method for confirming the presence of PFO,4,5 but ultrasound technology has made available many non-invasive techniques for diagnosing a right-to-left shunt (RLS), such as transthoracic echocardiography (TTE), transesophageal echocardiography (TEE) and transcranial Doppler ultrasound (TCD).6 TEE with bubble study is the most accepted standard noninvasive method for PFO diagnosis. It enables quantification of shunt size, documentation of anatomic characteristics and differentiation between PFO, atrial septal defect and pulmonary shunt.4,5 TCD is a more sensitive technique for shunt detection but less specific due to its inability to differentiate between cardiac and pulmonary shunting; it carries a sensitivity of 97% and specificity of 93% compared with TEE bubble study as the reference.7
Despite the efficacy of PFO occluder devices, their use has a rare potential risk of early and late complications including, in extreme cases, device dislodgement, atrial wall erosion, perforation, fracture, migration or embolization, infection, thrombosis, induction of arrhythmias and even death. Additionally, encumbrance of the interatrial septum by the prosthetic device may hinder future transseptal puncture and left-sided interventions such as left atrial appendage closure, arrhythmia ablation and mitral valve interventions. Finally, the risk of allergic reactions to nickel mesh cannot be excluded, and the need for prolonged dual antiplatelet therapy after the procedure may not be tolerated by all patients.8
Recently, a new percutaneous ‘deviceless’ system based on surgical suture-mediated PFO closure has been introduced in interventional practice, and shows a favorable efficacy and safety profile. The possible advantages of such a procedure are self-evident, notably avoidance of early and late complications related to the absence of a permanent implanted cardiac device. The complete and effective closure rates reported with this technique were similar to other device trials.8
ObjectivesThe aim of the present study is to present the procedural details of the NobleStitch® technique and to report the baseline characteristics of patients who underwent the procedure in our center.
MethodsStudy populationA single-center prospective observational registry was established between February 2020 and February 2021 (one year) to assess the safety, efficacy and possible advantages of a novel percutaneous suture-based PFO closure system (NobleStitch® EL; HeartStitch, Inc., Fountain Valley, CA, USA).
Patients with cryptogenic stroke, transient ischemic attack (TIA) or platypnea-orthodeoxia syndrome (POS) were referred to our center for PFO closure after neurology and cardiology assessment. The diagnosis of cryptogenic stroke or TIA was established after a large number of exams were performed including brain tomography and/or magnetic resonance, 24-hour Holter ECG monitoring, supra-aortic vessel Doppler ultrasound and TTE and/or TEE. Following this thorough assessment, the Risk of Paradoxical Embolism (RoPE) score was calculated for all patients.
Patients eligible for NobleStitch® PFO closure were selected after TEE assessment of interatrial septum anatomy, which included determining the presence of atrial septal aneurysm (ASA) (defined as an abnormally redundant interatrial septum with an excursion of >10 mm into the right or left atrium); RLS spontaneously or after Valsalva maneuver and appearance of microbubbles; presence of other atrial septal defects; and the anatomic characteristics of the PFO.
Patients with atrial septal defects other than PFO, complex PFO anatomies such as anterior location or fenestrated PFO or with poor quality TEE images, were excluded from the procedure.8
The statistical analysis was conducted using Excel® software. Descriptive statistics for baseline patient characteristics and procedural variables were calculated and are presented as mean or median, based on normal or non-normal distribution, respectively.
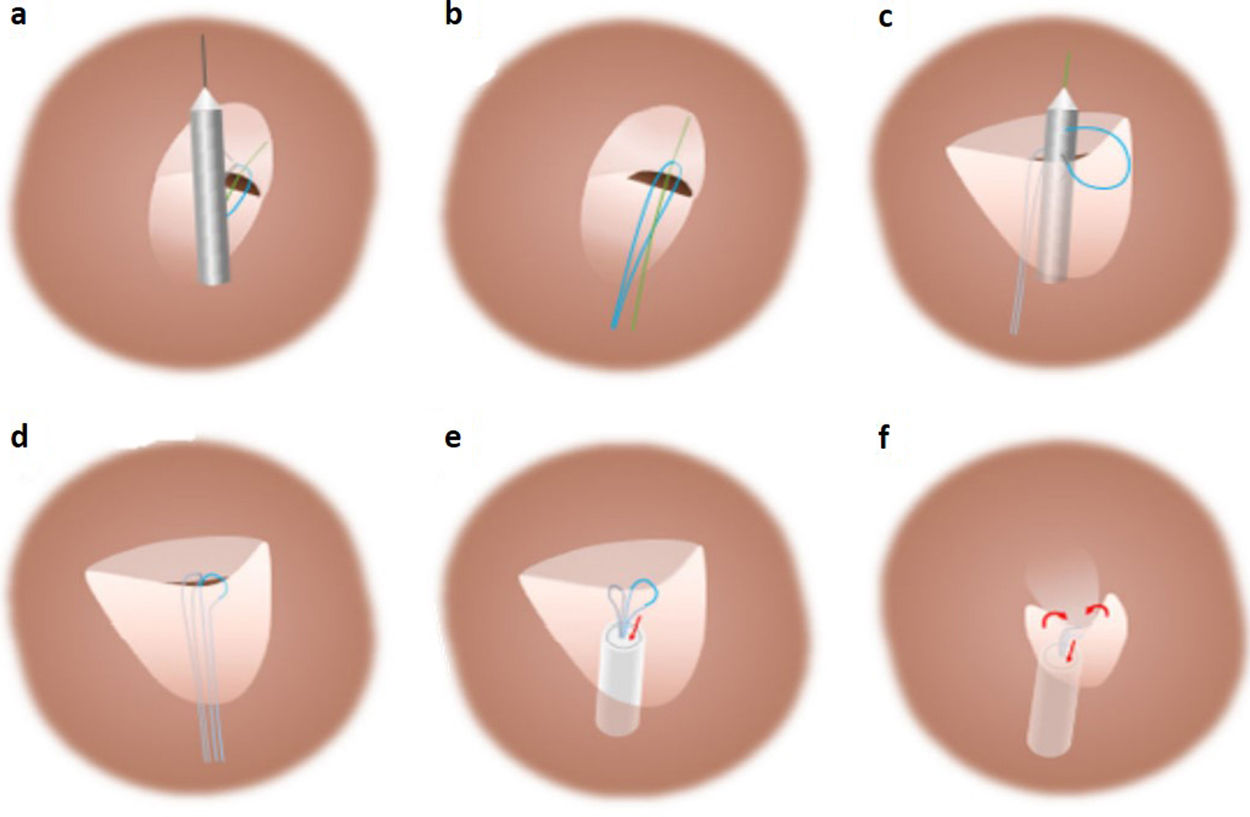
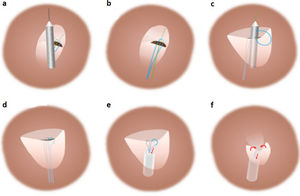
Description of the techniqueThe technique was implemented with the NobleStitch EL® system (HeartStitch Inc.), which is composed of three elements inserted sequentially through a femoral vein access. The procedure has been described in detail previously.8 Briefly, the NobleStitch EL® system consists of two dedicated suture delivery catheters that capture and suture the septum secundum and the septum primum using a 4-0 polypropylene suture which produces an S-shaped closure of the PFO (after contrast-enhanced balloon-mediated PFO anatomy assessment and proper placement of a 0.032́́ wire in the superior pulmonary vein and a 0.018́́ wire in the right innominate vein).9 Contrast injections are carried out according to operator discretion to obtain optimal engagement of each septum. A third element, the KwiKnot® catheter (HeartStitch, Inc.), is advanced over the septum secundum and septum primum sutures to approximate both septa, achieving closure by securing the stitch and trimming the excess suture material. Maintaining the tension on the sutures, the KwiKnot® delivery catheter is then used to advance and release a radiopaque polypropylene knot on the right side of the interatrial septum and to cut the proximal suture (Figures 1 and 2). Contrast injection was performed in all patients to assess the acute result. All procedures were carried out under fluoroscopic guidance providing direct visualization, usually without transesophageal or intracardiac echocardiographic monitoring. All patients were pre-treated with antiplatelet therapy (mostly aspirin 100 mg daily) or anticoagulation if clinically indicated. Patients received 100 IU/kg of heparin at the beginning of the procedure, followed by further boluses if needed, to maintain a constant activated clotting time of >250 s. After the procedure, continuation of antiplatelet therapy was left to the discretion of the attending physician. In the absence of other indications, the standard protocol was aspirin 100 mg daily for one month.8
Standard percutaneous suture-mediated patent foramen ovale closure with the NobleStitch® EL system. After placement of a 0.032́́ wire in the left superior pulmonary vein and a 0.018́́ wire in the superior vena cava, the NobleStitch secundum and primum catheters are sequentially advanced to suture (a) the septum secundum and (c) the septum primum, respectively. (b, d) After each NobleStitch needle firing, the delivery system is removed providing a long loop of suture through each septum. Finally, the suture ends are pulled to bend the septum primum towards the right atrium and close the PFO. At the same time, the KwiKnot delivery system is advanced to release a polypropylene knot at the right side of the interatrial septum and trim the excess thread (e, f) (drawing and description adapted from Gaspardone et al.8).
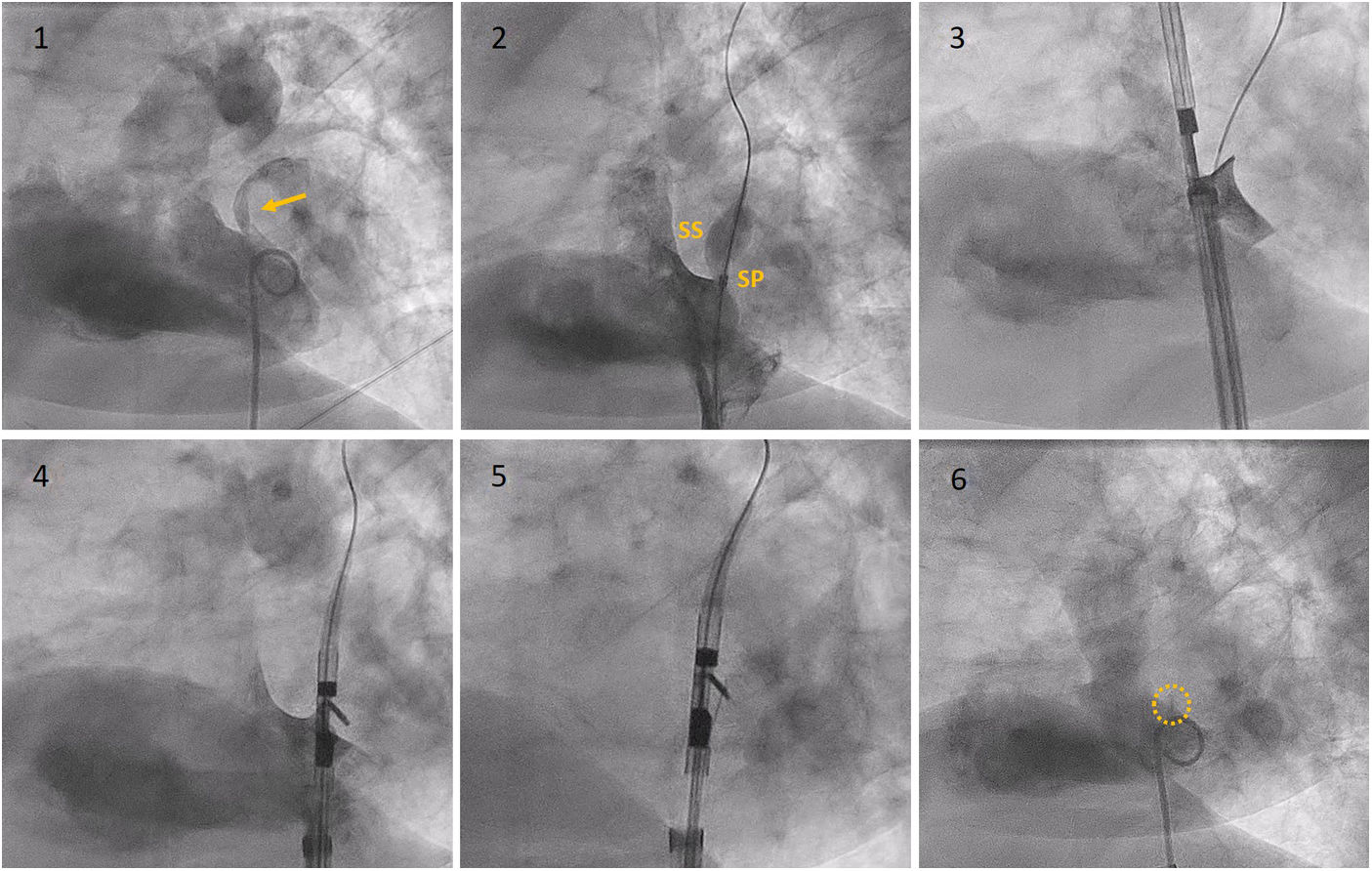
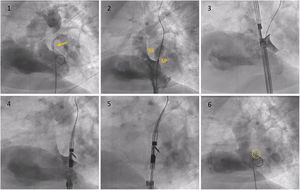
Fluoroscopy sequence of PFO closure with the NobleStitch® EL system. (1) Initial angiography showing contrast passage (yellow arrow) through the PFO. (2) Sizing balloon interrogation during contrast injection of the PFO outlines the septum secundum and septum primum anatomy. After placement of a 0.032́́ wire in the left superior pulmonary vein and a 0.018́́ wire in the superior vena cava, the NobleStitch secundum (3) and primum (4) catheters are sequentially advanced to suture the septum secundum and the septum primum (5), respectively. Contrast may be injected to help optimal engagement of each septum. After each needle firing, the delivery system is removed, providing a long loop of suture through each septum. The delivery system is advanced to release a polypropylene knot (6; yellow circle) at the right side of the interatrial septum and trim the excess thread. SP: septum primum; SS: septum secundum.
Between February 2020 and February 2021, 23 patients were considered eligible for suture-mediated PFO closure. It is important to note that in this period many elective procedures in which PFO closure was included were postponed due to the COVID-19 pandemic. In this period two patients with PFO referred for closure were not considered suitable for percutaneous suture-based PFO closure, one due to uninterpretable poor-quality TEE images, and the other due to a complex PFO anatomy.
The general characteristics of the study population are reported in Table 1. Patients selected for PFO closure were more frequently women (69.5%) and had a mean age of 51 years. Despite a high RoPE score (mean 6.7), a significant number of patients still had cardiovascular risk factors, with dyslipidemia being the most prevalent (60.1%). The majority of patients (91.3%) had a history of cryptogenic stroke, the other two being referred for TIA and POS.
Characteristics of the study population.
| Gender (female/male) | 16 (69.5%)/7 (30.5%) |
| Age, years | 51±13.2 |
| Cardiovascular risk factors | |
| Dyslipidemia | 14 (60.1%) |
| Hypertension | 5 (21.7%) |
| Diabetes | 0 (0%) |
| Smoking | 8 (34.8%) |
| Oral contraceptives | 4 (25%) |
| Venous thromboembolism | 1 (4.5%) |
| PFO closure indication | |
| Cryptogenic stroke | 21 (91.3%) |
| TIA | 1 (4.3%) |
| POS | 1 (4.3%) |
| RoPE score | 6.7 |
PFO: patent foramen ovale; POS: platypnea-orthodeoxia syndrome; TIA: transient ischemic attack.
The functional and anatomical characteristics of the PFOs in these 23 patients are described in Table 2.
Functional and anatomical characteristics of patent foramen ovale.
| Spontaneous shunt, n (%) | 20 (86.9%) |
| Shunt during Valsalva only, n (%) | 3 (13.1%) |
| Tunnel-like, n (%) | 12 (52.2%) |
| PFO diameter, mm | 2.9±1.0 |
| PFO length, mm | 10.2±6.1 |
| Atrial septum aneurysm, n (%) | 9 (39.1%) |
PFO: patent foramen ovale.
PFO closure with the NobleStitch® system was successfully performed in all 23 patients. All procedures were performed under local anesthesia and fluoroscopic monitoring. TEE was needed in one procedure to help guidewire passage through the PFO. The introducers used for femoral vein access were all 14F size (maximum final diameter) and there was no need for a second vascular access. There were no clinical consequences, including vascular complications.
The mean duration of the procedure was 52 min. The earlier half of the procedures were significantly more prolonged (mean time 61.2 min) than the more recent ones (mean time 45 min) due to the learning curve of the technique. The fastest procedure was completed in only 35 min and the longest 97 min (the first). The mean contrast used was 187 ml (50 minimum and 225 maximum) and median radiation dose absorbed per patient was 61.5±68 Gy cm2.
At the end of all procedures the acute success criterion (no passage of contrast to the left atrium) was achieved.
All patients were discharged within 24 hours of the procedure, after undergoing TTE which excluded complications. No peri- or postprocedural arrhythmias were recorded.
Patients were scheduled for clinical and imaging assessment three months after the intervention, including TTE with bubble test study and TCD. If any of these exams showed positive findings, residual shunt were ruled out by TEE (Table 3).
Procedure characteristics.
| Proctored procedure, n (%) | 7 (30.4) |
| TEE guidance, n (%) | 1 (4.3) |
| Local anesthesia only, n (%) | 23 (100) |
| Mean fluoroscopy time, min | 15.2±5.5 |
| Mean procedure time, min | 52.9±17.1 |
| Radiation dose, Gy cm2 | 61.5±41.3 |
| Contrast medium, ml | 187±68 |
TEE: transesophageal echocardiography.
Atherosclerosis is by far the most frequent cause of brain ischemia; however, a significant number of ischemic strokes are due to cardioembolism, large vessel atherothromboembolism, small vessel occlusive disease or other less common mechanisms. The term cryptogenic stroke designates the category of ischemic stroke for which no probable cause is found despite a thorough diagnostic assessment and is defined in the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification as a stroke of undetermined etiology.2 Patients presenting with this clinical picture should be screened for the presence or absence of a PFO. Recently, the European position paper on PFO stated that when a PFO is thought likely to be implicated in a cryptogenic embolism, it should be classified as PFO-related instead of cryptogenic stroke.10
In the presence of a PFO, certain anatomic characteristics are associated with a higher risk of embolism, such as the presence of an ASA (also termed a hypermobile septum primum). As is known, changes in right atrial volume and pressure lead to moments of patency of the foramen ovale. The presence of an ASA may open the PFO with every heartbeat, thereby increasing the potential for thrombus passage from the venous to the arterial system. A similar effect is exerted by a Eustachian valve (or a Chiari network), which can direct blood flow from the inferior vena cava to the foramen ovale.3
A meta-analysis of five randomized clinical trials (n=3440) showed that percutaneous PFO device closure plus long-term medical antithrombotic (primarily antiplatelet) therapy reduced the risk of recurrent stroke compared with long-term medical antithrombotic therapy (antiplatelet or anticoagulant) alone. However, this meta-analysis also found a significantly increased risk of atrial fibrillation (AF) in patients with devices, the risk being device-dependent (the great preponderance of AF events were transient episodes occurring within 4-6 weeks of device placement). Occurring in 3.2% of patients undergoing a device procedure, these generally self-limited, periprocedural AF events have less likelihood of serving as a new stroke source compared with AF events that occur later, and their clinical consequences are yet to be clarified.11,12 Observational studies have reported chest pain as an occasional side effect associated with device implantation, thought to be secondary to an enhanced inflammatory response, in some cases due to nickel allergy.8 An observational survey of approximately 14000 PFO device implants worldwide reported an incidence of 1 in 500 implantations resulting in surgical removal, most commonly due to severe and persistent chest pain, thought to be caused by allergy-induced formation of excessive scar tissue in 50% of cases.13
Percutaneous ‘deviceless’ systems were recently introduced with the purpose of overcoming most of the limitations of traditional PFO occluders. This new technique is potentially superior to traditional closure systems, particularly in terms of device-related complications such as dislodgement, fracture, embolization, migration, atrial wall erosion, heart perforation, infection, induction of atrial arrhythmias and major changes in atrial anatomy and function.8
The NobleStitch EL Italian Registry8 has shown that suture-mediated PFO closure is feasible in the majority of septal anatomies, with septal suture using this system being successfully carried out in 186 of 200 (96%) patients, and provides effective PFO closure comparable to traditional devices: at 206±130 days of follow-up, contrast TTE with the Valsalva maneuver revealed no RLS in 75% of patients and RLS grade ≤1 in 89%; significant RLS (grade 2 and 3) was present in 11%, and the technique's safety profile was good at medium-term follow-up.
Our experience is in line with this registry, indicating that suture-mediated PFO closure represents a valid, feasible and safe alternative to traditional umbrella-like devices. Although this technique requires an additional amount of contrast medium and radiation dose, the facts that general anesthesia or sedation and echocardiographic monitoring are not required during the procedure, and that it is a deviceless technique, largely compensate for these drawbacks. Another important point is that, in the event of failure, this technique does not preclude the possibility of implanting an umbrella-like device, if needed.
While it is essentially a fluoroscopically guided procedure that does not require echocardiographic monitoring, it is extremely important to perform an accurate preprocedural TEE assessment to optimally define the anatomical features of the PFO. All of our patients accepted for the procedure had a TEE assessment with good visualization of the interatrial septum, the PFO and adjacent structures.
A modified technique using a second NobleStitch® primum catheter has been described, which aims to increase the adhesion surface of the septa in order to maximize the success rate of the procedure, especially for very large tunnels and floppy aneurysms. Nevertheless, this alternative technique still needs to be tested in a larger number of cases and was not used in any of our patients.9
The majority of cases were performed recently, thereby precluding the accurate reporting of follow-up, which is why the aim of this paper was to document our initial experience with patient selection and with the technique itself. Experience with this new device is rapidly increasing in Europe and a clinical trial comparing PFO closure results with the NobleStitch EL® and with the FDA-approved Amplatzer PFO Occluder® device is currently underway (the NobleStitch EL STITCH trial).14 The results of this trial will allow a better understanding of the efficacy of this technique.
ConclusionsOur experience suggests that suture-mediated PFO closure represents a valid, feasible and safe alternative to traditional umbrella-like devices. It can be an option in the majority of PFO anatomies. However, the use of this technique as a first choice in PFO closure will require results of larger series and clinical trials.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Prof. Dr. Achille Gaspardone for allowing the use and editing of the schematic figures in this article.