A 70-year-old male patient with a history of rheumatic mitral valve disease, who underwent percutaneous mitral balloon valvuloplasty (2004) and surgical mitral valve repair (2007) was referred for left atrial appendage (LAA) closure due to LAA thrombus persistence, despite adequate anticoagulation.
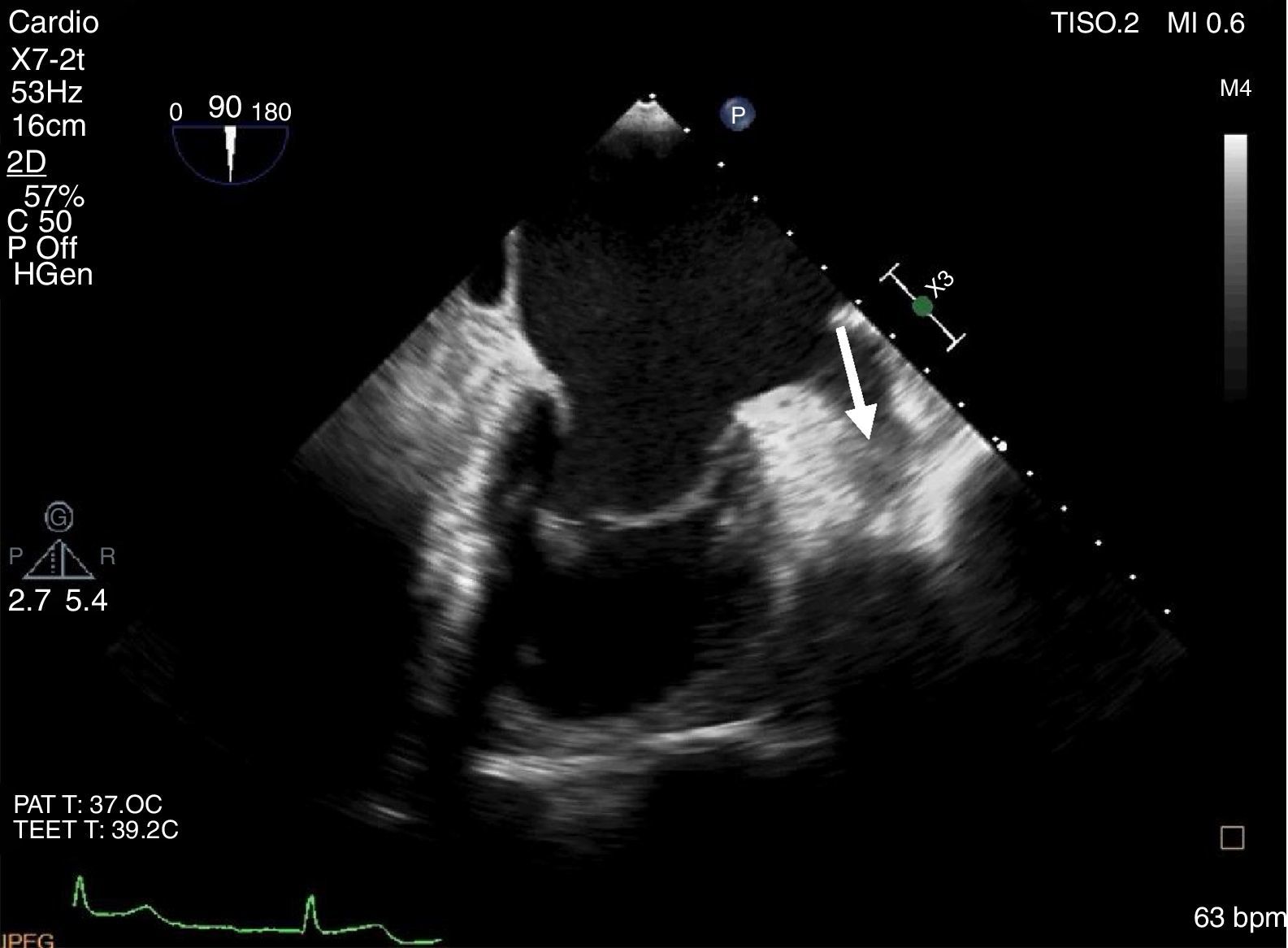
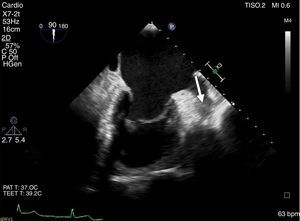
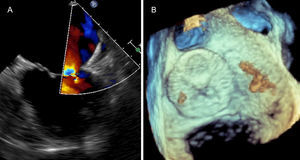
The LAA thrombus was documented in a transesophageal echocardiography (TEE) (Figure 1). This thrombus was formed despite long-term oral anticoagulation with warfarin. The first approach was to increase the anticoagulation therapeutic range (INR 3.0-4.0) for 3 months, followed by high-dose enoxaparin (1.5 mg/kg, every 12 h) with limited success.
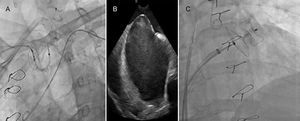
Due to high cardioembolic risk, the patient underwent successful LAA device implantation (Amulet™ St. Jude Medical; 28 mm) guided by intracardiac echocardiography (ICE), together with a cerebral protection system (Sentinel™, Claret Medical) (Figure 2). The patient received five days of unfractionated heparin prior to the procedure to reduce the “thrombotic burden”, resulting in partial reduction of the thrombus. Using ICE negated the need for sedation or intubation, thus permitting continuous monitoring and early management of acute neurologic complications.
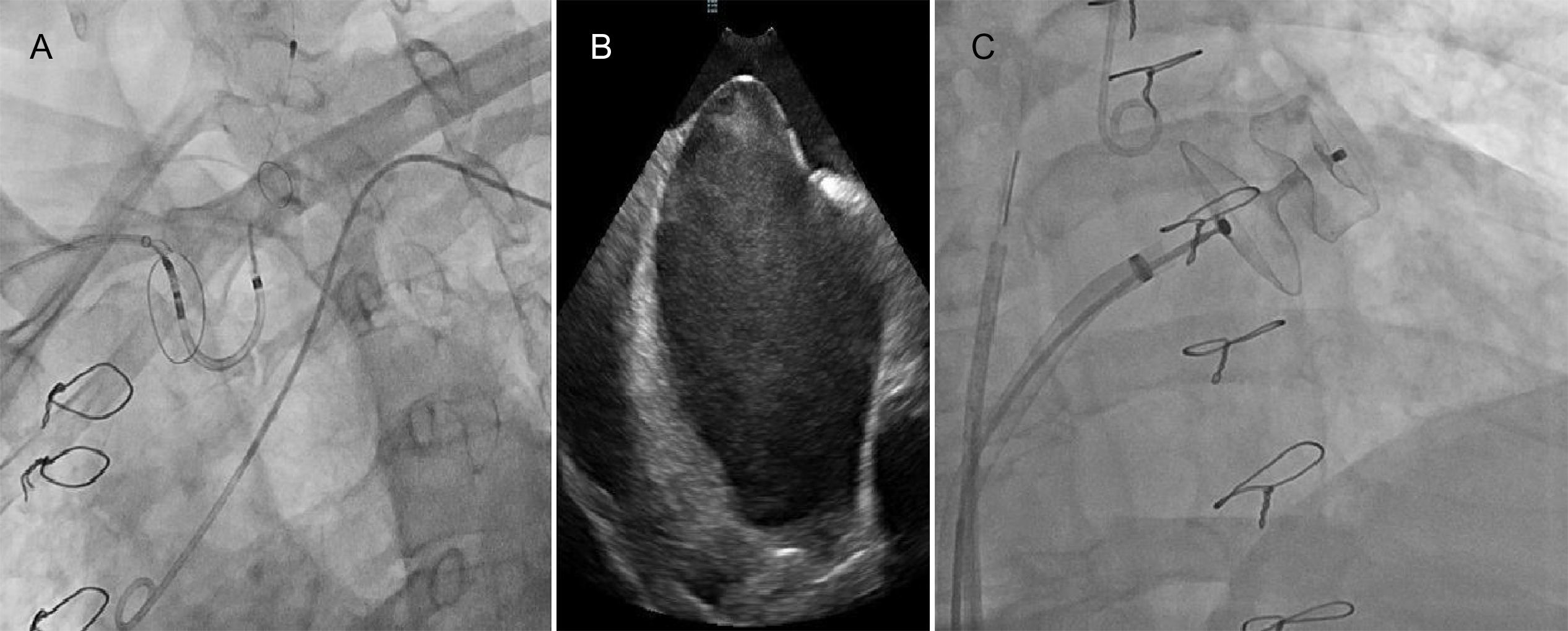
Device implantation using a cerebral protection system guided by intracardiac echocardiography (ICE).
(A) Fluoroscopy showing the cerebral protection system (Sentinel™, Claret Medical). (B) Transseptal puncture guided by ICE. (C) Fluoroscopy showing the device (Amulet™ St. Jude Medical; size 28 mm) implantation.
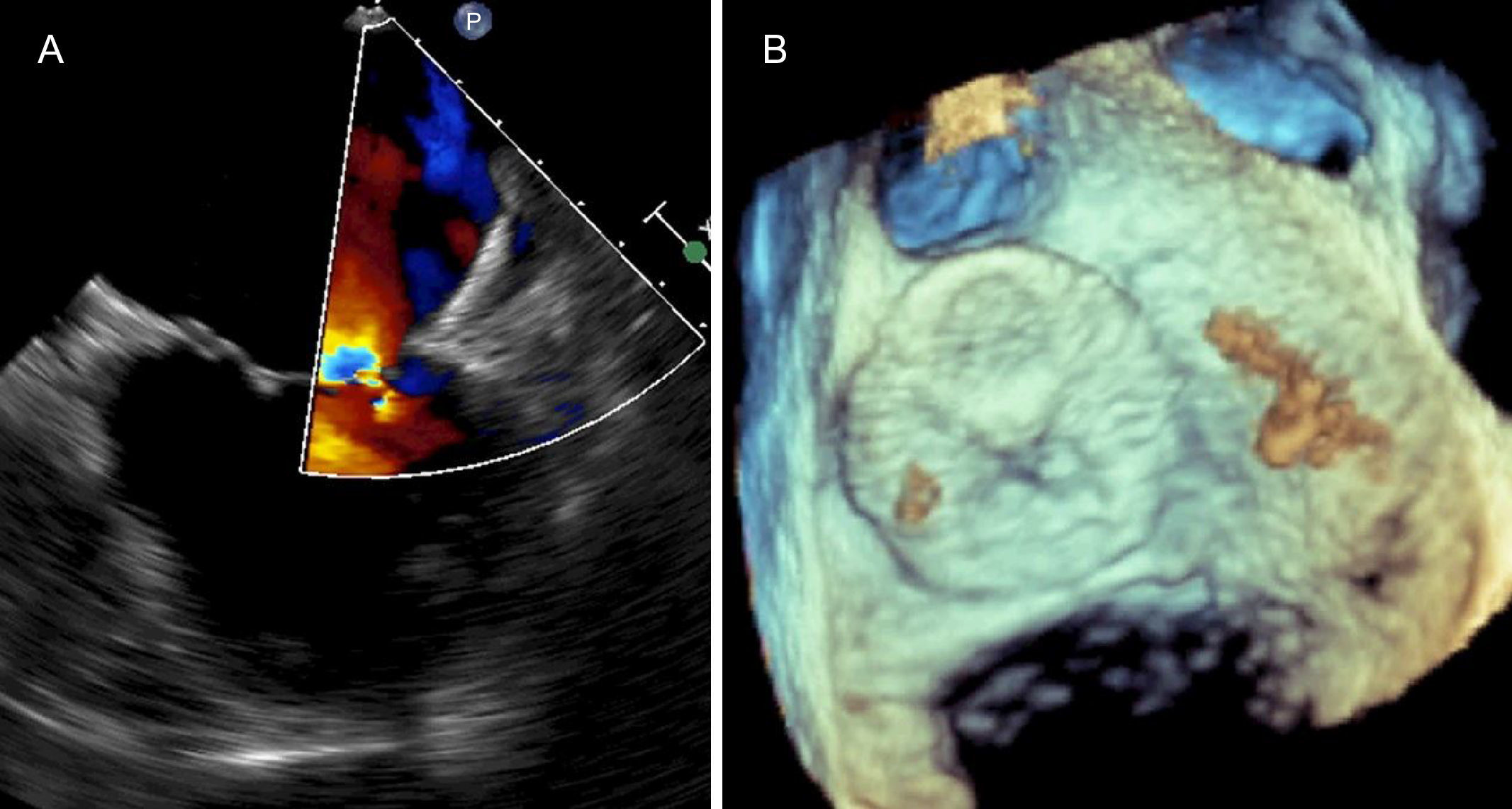
The procedure was performed without complications and no thrombotic material was retrieved from the cerebral protection system. The patient was discharged the following day on warfarin. The follow-up TEE at four weeks showed the device appropriately implanted (Figure 3).
It is crucial to take into account strategies to prevent thrombus migration in these circumstances such as not injecting contrast directly into the LAA, performing the cannulation of the delivery system without guide or pigtail and using a cerebral protection system.
Conflicts of interestThe authors have no conflicts of interest to declare.