Pregnant women with mechanical prosthetic heart valves are at increased risk for valve thrombosis. Management decisions for this life-threatening complication are complex. Open-heart surgery has a very high risk of maternal mortality and fetal loss. Bleeding and embolic risks associated with thrombolytic agents, the limited efficacy of thrombolysis in certain subgroups, and a lack of experience in the setting of pregnancy raise important concerns.
Case reportWe report a case of mitral prosthetic valve thrombosis in early pregnancy, which was successfully treated with streptokinase. Ten years later, the same patient had an uneventful pregnancy, throughout which acenocoumarol was maintained.
ConclusionWith this case we review the prevention (with oral anticoagulant therapy) and treatment of prosthetic valve thrombosis during pregnancy, which is important for both obstetrician and cardiologist.
Uma doente grávida com uma prótese mitral mecânica tem risco aumentado de trombose de prótese. Esta complicação potencialmente fatal obriga a decisões terapêuticas complexas. A cirurgia cardíaca tem um risco muito elevado de mortalidade materna e fetal. Os riscos hemorrágico e embólico associados aos agentes trombolíticos, a eficácia limitada da trombólise em alguns subgrupos de doentes e a falta de experiência existente no contexto de gravidez são uma forte preocupação.
Caso clínicoOs autores descrevem um caso de uma doente com trombose de prótese mitral no primeiro trimestre de gravidez, tratada com sucesso com estreptoquinase. Dez anos mais tarde, a mesma doente tem uma gravidez não complicada sob tratamento com acenocumarol.
ConclusãoEste caso permite uma revisão da prevenção (anticoagulação) e do tratamento de trombose de prótese durante a gravidez.
Pregnant women with mechanical prosthetic heart valves are at increased risk for prosthetic valve thrombosis due to both higher levels of coagulation factors and reduced endogenous fibrinolytic capacity. Management decisions for this life-threatening complication are complex. Open-heart surgery has a very high risk of maternal mortality and fetal loss, especially in early pregnancy. Furthermore, bleeding and embolic risks associated with thrombolytic agents, the limited efficacy of thrombolysis in certain subgroups, and a lack of experience in the setting of pregnancy raise important concerns.
Case reportA 25-year-old woman with a mechanical mitral valve prosthesis (St. Jude Medical) and a 14-week pregnancy was admitted to our cardiac intensive care unit due to orthopnea and exertional dyspnea (NYHA functional class III) of 36 hours’ duration.
Her past cardiac history began at the age of 10, when she was diagnosed with rheumatic mitral valve disease while being evaluated for fever of unknown origin and signs of heart failure. Following the diagnosis, rheumatic fever prophylaxis was instituted with 1.2 million units of benzathine penicillin intramuscularly every four weeks. Two years later, she was referred to our institution and hospitalized due to decompensated heart failure. Investigation of her heart failure confirmed rheumatic mitral and tricuspid valve disease with severe mitral regurgitation due to anterior leaflet prolapse, severe tricuspid regurgitation, and pulmonary hypertension. She underwent surgical repair of both mitral (Carpentier-Edwards ring) and tricuspid (de Vega technique) valves. Three weeks later, the patient was reoperated due to failure of the mitral valve repair procedure and persistent heart failure. A biological prosthesis was placed in the mitral position and a redo of the tricuspid repair was performed with a ring. Methycillin-resistant Staphylococcus epidermidis was isolated through bacteriological examination of the excised mitral valve and ring. The second surgery was complicated by mediastinitis and acute renal failure, and prolonged overall hospitalization (she was discharged nearly three months after the initial surgery). After a symptom-free period of three years, at the age of 15 she developed rapidly progressive clinical manifestations of heart failure and was found to have thickening and calcification of the mitral prosthesis, causing severe stenosis. The biological mitral prosthesis was replaced with a mechanical prosthesis (25-mm St. Jude Medical) and the postoperative course was uneventful. Subsequently, the patient had normal functional capacity, without clinically relevant cardiovascular events, and was simply managed with adjusted-dose warfarin therapy.
The patient's past obstetric history included two unsuccessful pregnancies. The first, at the age of 19, terminated with elective abortion at week 10 due to fetal malformation; the second, at age 22, was complicated with unexplained in-utero embryo death at week 7.
For the current pregnancy, low molecular weight heparin (LMWH) was substituted for warfarin therapy at week 6, and continued up to the time of presentation. The regimen consisted of nadroparin calcium 5700 anti-Xa IU (in our institution, nadroparin was the most commonly used LMWH for bridging anticoagulation) administered subcutaneously every 12 hours. Compliance with LMWH therapy was 100% throughout this period.
The physical exam on presentation showed hyperpnea, intolerance to prolonged (>10 min) supine position, jugular venous distension, regular tachycardia (125 bpm), blood pressure 100/60 mmHg, dull prosthetic closing click, grade 2/6 mid-diastolic apical murmur, and inspiratory crackles heard bilaterally over the lower lung fields. The ECG documented sinus tachycardia and diffuse ST-T abnormalities.
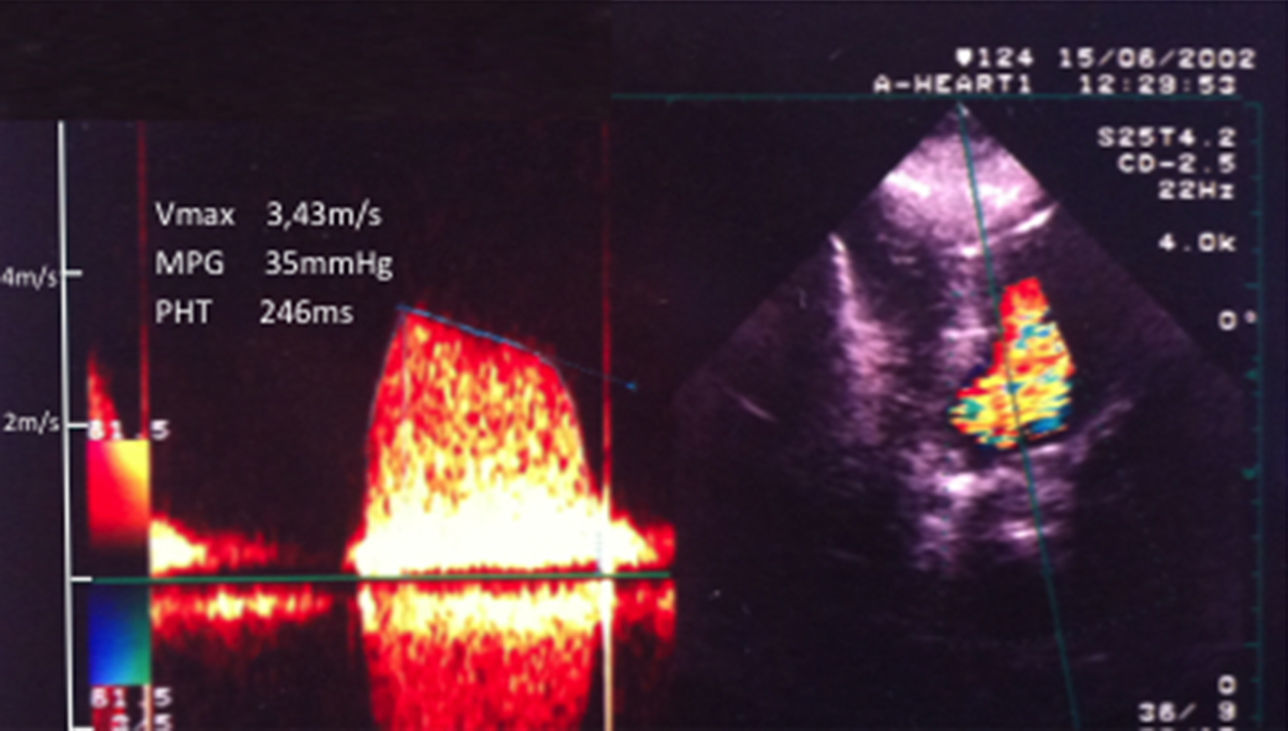
Transthoracic echocardiography (TTE) showed inadequate mobility of one of the prosthetic leaflets. The M-mode recording at the level of the prosthetic leaflets in parasternal long-axis view showed rounded edges. Peak and mean transprosthetic gradients were 48 and 35 mmHg, respectively, and mitral valve area (pressure half-time method) was 0.8 cm2 (Figure 1). Other echocardiographic findings included mild mitral regurgitation, mild tricuspid regurgitation, and severe pulmonary hypertension (93 mmHg). An attempt to perform a full transesophagoeal echocardiographic evaluation was unsuccessful due to patient discomfort and breathlessness. Nevertheless, no images consistent with large left-sided thrombus were recorded.
After a multidisciplinary evaluation of the available treatment options, thrombolysis was promptly performed. Streptokinase was administered using a loading dose of 250000 IU given over 30 minutes, followed by 100000 IU/h infusion for six hours.
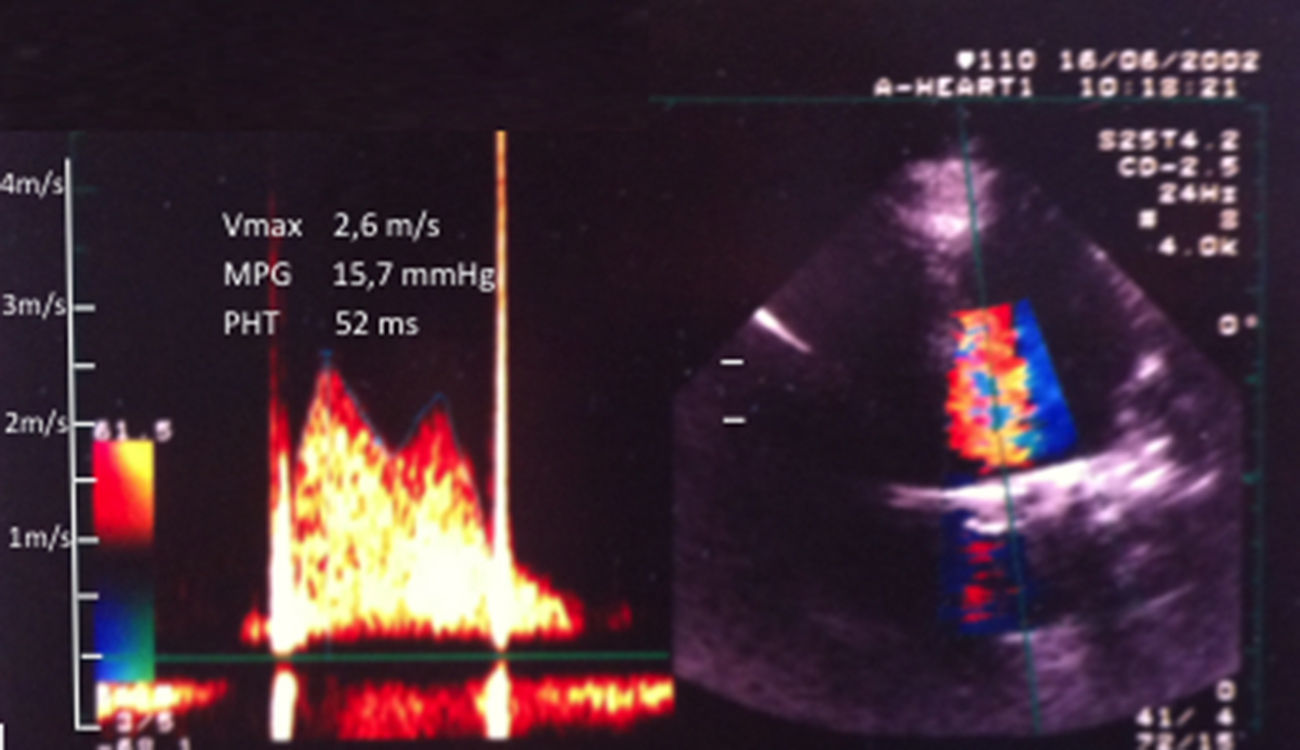
Clinical course and serial echocardiographic findingsThe first echocardiographic evaluation performed during thrombolysis was obtained approximately two hours after the start of treatment. Compared to baseline, the mean transprosthetic gradient (25 mmHg) and systolic pulmonary pressure (78 mmHg) were mildly reduced. Significant and sustained improvement in clinical manifestations was noted only four hours after the start of streptokinase. An hour later, the patient spontaneously reported complete resolution of the dyspnea, and her vital signs and cardiac auscultation were normal. At that time, a second echocardiogram was performed, which showed apparently normal excursion of both prosthetic leaflets, regression of the transprosthetic gradients to values similar to those obtained prior to pregnancy, and disappearance of the mitral and tricuspid regurgitation. Figure 2 shows the transprosthetic flow pattern on the second day of hospitalization.
Two adverse events occurred during thrombolytic treatment. Mild gingival bleeding was noticed early during treatment. Three hours after treatment initiation, the patient complained of lower abdominal pain radiating to the lumbar region. Abdominal and pelvic ultrasonography ruled out retroplacental bleed, and showed that the fetus maintained normal vital status.
Overall, streptokinase was infused for six hours, and was stopped when the clinical and echocardiographic reassessments were unremarkable. Intravenous unfractionated heparin (UFH) was started two hours later. This timing was chosen based on close monitoring of activated partial thromboplastin time (aPTT) values after streptokinase was stopped. The UFH infusion rate was guided by aPTT monitoring, and the therapeutic goal was set to an aPTT value at least twice the control. Warfarin was started on the evening of the second day of hospitalization, and the dose was targeted to achieve an INR between 2.5 and 3.5 (mean daily dose of 6.25 mg up to 36 weeks gestation). A transesophagoeal echocardiogram was electively performed on day 10, and ruled out prosthetic malfunction. The patient was discharged on day 11.
Intravenous UFH was substituted for warfarin therapy at 36 weeks gestation. A normal healthy child was born after 37 weeks gestation by elective cesarean section.
Over the following ten years, the patient had two further pregnancies while on warfarin therapy, both ending with miscarriage. She was requiring a mean daily dose of 6.25 mg warfarin for adequate INR control.
At the age of 35, the patient had a sixth pregnancy. Prior to pregnancy, warfarin was switched to acenocoumarol, since the teratogenic risk of the daily dose of warfarin was considered to be very high. The mean daily dose requirement for acenocoumarol was 3 mg, and the same dose was required during pregnancy. This pregnancy was uneventful. Three days prior to delivery, acenocoumarol was bridged to intravenous UFH. The delivery had no complications and a healthy child was born after 38 weeks gestation. The patient has been on acenocoumarol since then.
DiscussionThis case demonstrates that mechanical prosthetic valve thrombosis occurring during early pregnancy may be successfully treated using a thrombolytic agent. Pregnancy in a patient with a mechanical prosthesis is associated with maternal mortality between 2% and 15%, mainly due to valve thrombosis while on heparin therapy.1
Pregnancy is a relative contraindication for the administration of thrombolytic agents, due to the risk of embolization (10%) and subplacental bleeding,2 a life-threatening complication for both mother and fetus. Reported experience with the use of these agents during pregnancy is limited to a small number of cases and clinical settings. Because fetal loss is high with surgery, fibrinolysis may be considered instead of surgery in non-critically ill patients when anticoagulation fails.2 Fibrinolysis should be applied in critically ill patients when surgery is not immediately available2 and is the therapy of choice in right-sided prosthetic valve thrombosis. Streptokinase has been used for the treatment of deep venous thrombosis, pulmonary embolism, middle cerebral artery occlusion, and prosthetic valve thrombosis.
The management of pregnant patients with mechanical prosthetic valves involves several complex issues. Vitamin K antagonists between the sixth and the twelfth weeks have been associated with an increased risk; the reported incidence varies between 0.6% and 10%2 for embryopathic effects (because the drugs cross the placenta) or early abortion, and the risk is dose-related3 (it is relatively low in patients taking ≤5 mg warfarin per day4). Delivery without prior suspension of oral anticoagulation (OAC) is associated with an increased risk of bleeding in the neonate.2
UFH and LMWH do not cross the placenta and there is thus no risk of embryopathy; they may be used during pregnancy in patients with mechanical prosthetic valves, especially during the first 12 weeks of gestation. The superiority of either UFH or LMWH in the first trimester is unproven. Long-term heparin therapy is difficult to manage and the incidence of prosthetic thrombosis appears to be considerably higher with heparin than with vitamin K antagonists, even when using adjusted doses of the former.1 LMWH has the advantage of providing a more stable antithrombotic effect; nevertheless, experience with administration during pregnancy in patients with mechanical heart valves is limited, and weekly monitoring of anti-factor Xa levels is required2 to adjust the dose in order to achieve therapeutic levels of anticoagulation. The efficacy of LMWH during pregnancy has been demonstrated in the prevention and treatment of venous thromboembolism.
For the first trimester in patients with mechanical heart valves, discontinuation of OAC between weeks 6 and 12 and a switch to adjusted-dose UFH (aPTT ≥2 times control; in high-risk patients applied as intravenous infusion) or LMWH (twice daily with dose adjustment according to body weight and to target anti-Xa level of 0.8–1.2 U/ml at 4–6 hours post-dose) should be considered in patients with a daily dose requirement of >5 mg for warfarin or >2 mg for acenocoumarol.2
Nevertheless, there is no consensus regarding antithrombotic treatment during the first trimester of pregnancy in patients with mechanical heart valves who require a mean daily dose of <5 mg warfarin or <2 mg acenocoumarol for adequate anticoagulation. Some prefer to continue vitamin K antagonists, but others switch to subcutaneous UFH or LMWH, particularly between weeks 6 and 12. The choice should be made after clearly informing the patient and her partner of the risks inherent in the different strategies. The use of warfarin throughout pregnancy provides the best maternal protection1,3 but has been associated with a high rate of fetal loss (including miscarriage, still-birth, and neonatal death), besides the risk of embryopathy.5 The strategy of administering heparin during the first trimester followed by a vitamin K antagonist is associated with more than double the risk of maternal thromboembolic complications or death, compared to warfarin throughout pregnancy.1
However, there is general agreement regarding treatment after the first trimester. The usual recommendation is that vitamin K antagonists be used, and replaced by subcutaneous or intravenous heparin at the 36th week2 to avoid the risk of neonatal intracranial hemorrhage during delivery. The switch of anticoagulation regimens during pregnancy should be implemented in a hospital.2 If delivery starts while on OAC, cesarean delivery is indicated.2 In patients with a high risk of valve thrombosis, a planned cesarean section may be considered (keeping the time without OAC as short as possible).2
In most studies of pregnant patients on vitamin K antagonists, warfarin was being taken. Hence, there are more reports on switching warfarin to heparin than for acenocoumarol. In the case of our patient, because of the daily dose requirement of >5 mg warfarin, which is known to be more teratogenic,3,4 acenocoumarol was substituted for warfarin in the last pregnancy. It is estimated that a mean daily dose of 5 mg warfarin has the same anticoagulant effect (measured by INR levels) as a mean daily dose of 2 mg acenocoumarol.6 This is the rationale for the guideline recommendation regarding the cutoff value (2 mg per day) for deciding on whether to maintain acenocoumarol or to switch to heparin between weeks 6 and 12 of gestation.2 Nevertheless, it should be noted that there are no dose-effect studies on the teratogenic potential of acenocoumarol. Our patient had four miscarriages while on warfarin therapy, and an uneventful pregnancy on a mean daily dose of 3 mg acenocoumarol.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.