Left bundle branch block (LBBB) is a frequent finding in patients with heart failure (HF), particularly in those with dilated cardiomyopathy (DCM). LBBB has been commonly described as a consequence of DCM development. However, a total recovery of left ventricular (LV) function after cardiac resynchronization therapy (CRT), observed in patients with LBBB and DCM, has led to increasing acknowledgement of LBBB-induced dilated cardiomyopathy (LBBB-iDCM) as a specific pathological entity. Its recognition has important clinical implications, as LBBB-iDCM patients may benefit from an early CRT strategy rather than medical HF therapy only. At present, there are no definitive diagnostic criteria enabling the universal identification of LBBB-iDCM, and no defined therapeutic approach in this subgroup of patients. This review compiles the main findings about LBBB-iDCM pathophysiology and the current proposed diagnostic criteria and therapeutic approach.
O bloqueio de ramo esquerdo (BRE) é encontrado frequentemente em doentes com insuficiência cardíaca (IC), particularmente naqueles com miocardiopatia dilatada (MCD). O BRE tem sido descrito sobretudo como uma consequência no decorrer da história natural de alguns doentes com MCD. No entanto, a observação de uma recuperação total da função do ventrículo esquerdo (FVE) após terapia de ressincronização cardíaca, tem contribuído para o reconhecimento crescente da MCD induzida por BRE como uma entidade patológica específica. Esta definição tem implicações clínicas importantes, uma vez que os doentes com este diagnóstico podem beneficiar de uma estratégia precoce de terapia de ressincronização, além da terapêutica médica estabelecida para a IC. Até ao momento, não existem critérios de diagnóstico específicos que permitam uma identificação universal de doentes com MCD induzida por BRE, nem uma abordagem terapêutica definida. Esta revisão sumariza a evidência existente acerca das principais caraterísticas da fisiopatologia da MCD induzida por BRE, bem como os critérios de diagnóstico que têm sido propostos para a sua identificação e estratégias de abordagem terapêutica.
Left bundle branch block (LBBB) is a common electrocardiographic finding, which has been extensively associated with poor clinical outcomes such as left ventricular (LV) dysfunction development.1,2 The presence of LBBB in patients with dilated cardiomyopathy (DCM) is associated with poor prognosis, specifically increased morbidity and mortality.3 DCM is defined as LV or biventricular dilatation and LV dysfunction in the absence of overload conditions or coronary artery disease (CAD).4 Classically, LBBB has been described as a consequence of DCM development, but the presence of chronic LBBB has also been associated with LV desynchrony and subsequent dysfunction. This suggests a causative role for LBBB in LV remodeling and heart failure (HF) development.5–7 Several studies have showed poorer response and outcomes in patients with DCM and concomitant LBBB to the recommended guideline-directed medical therapy (GDMT), when compared with those without LBBB. Interestingly, these studies also revealed a more favorable response in terms of reverse remodeling, normalization of QRS duration and LV ejection fraction (LVEF) improvement when an early cardiac resynchronization therapy (CRT) strategy is adopted.8–10 In this context, many of these patients have been described as hyper-responders to CRT, leading to the emergence of a new pathological entity: LBBB-induced DCM (LBBB-iDCM).7
In this review, we aim to summarize the existing evidence about LBBB-iDCM pathophysiology, diagnosis, and therapeutic approach described in the literature to date. Our main goal is to contribute to greater knowledge of this still poorly understood and studied entity.
We performed a literature search in the MEDLINE (through PubMed) electronic database. Search terms included left bundle branch block, cardiomyopathy, dilated cardiomyopathy, dessynchronopathy, left bundle branch block-induced cardiomyopathy.
Left bundle branch block definitionLeft bundle branch block definition prevalence in the general population has been described as 0.1–1.1%11–13 and as 20–30% in HF patients.14 In asymptomatic adults, LBBB is more frequently documented in men than in women,15 and its prevalence progressively increases with age.16
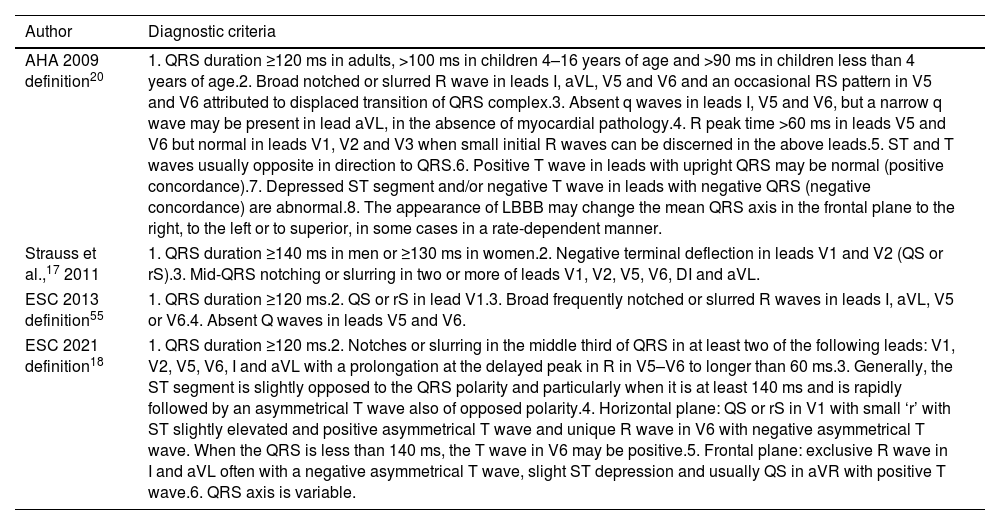
Several diagnostic criteria have been proposed for the identification of LBBB (Table 1). In 2011, Strauss et al.17 proposed strict criteria for LBBB diagnosis, suggesting that only patients with complete LBBB would truly benefit from CRT. The most recent LBBB diagnostic criteria18 are described in Table 1, highlighting the importance of features such as QRS notching/slurring, delayed R-wave peak time and ST segment and T-wave evaluation, for LBBB diagnosis.19 Calle et al.19 pointed to a possible LBBB underdiagnosis when employing the newest European Society of Cardiology (ESC) 2021 diagnostic criteria in comparison with the utilization of the previous ESC 2013 definition. The disparity is attributed to a more selective/strict criteria for LBBB diagnosis when applying the ESC 2021 definition.
Diagnostic criteria of left bundle branch block according to different scientific statements.
| Author | Diagnostic criteria |
|---|---|
| AHA 2009 definition20 | 1. QRS duration ≥120 ms in adults, >100 ms in children 4–16 years of age and >90 ms in children less than 4 years of age.2. Broad notched or slurred R wave in leads I, aVL, V5 and V6 and an occasional RS pattern in V5 and V6 attributed to displaced transition of QRS complex.3. Absent q waves in leads I, V5 and V6, but a narrow q wave may be present in lead aVL, in the absence of myocardial pathology.4. R peak time >60 ms in leads V5 and V6 but normal in leads V1, V2 and V3 when small initial R waves can be discerned in the above leads.5. ST and T waves usually opposite in direction to QRS.6. Positive T wave in leads with upright QRS may be normal (positive concordance).7. Depressed ST segment and/or negative T wave in leads with negative QRS (negative concordance) are abnormal.8. The appearance of LBBB may change the mean QRS axis in the frontal plane to the right, to the left or to superior, in some cases in a rate-dependent manner. |
| Strauss et al.,17 2011 | 1. QRS duration ≥140 ms in men or ≥130 ms in women.2. Negative terminal deflection in leads V1 and V2 (QS or rS).3. Mid-QRS notching or slurring in two or more of leads V1, V2, V5, V6, DI and aVL. |
| ESC 2013 definition55 | 1. QRS duration ≥120 ms.2. QS or rS in lead V1.3. Broad frequently notched or slurred R waves in leads I, aVL, V5 or V6.4. Absent Q waves in leads V5 and V6. |
| ESC 2021 definition18 | 1. QRS duration ≥120 ms.2. Notches or slurring in the middle third of QRS in at least two of the following leads: V1, V2, V5, V6, I and aVL with a prolongation at the delayed peak in R in V5–V6 to longer than 60 ms.3. Generally, the ST segment is slightly opposed to the QRS polarity and particularly when it is at least 140 ms and is rapidly followed by an asymmetrical T wave also of opposed polarity.4. Horizontal plane: QS or rS in V1 with small ‘r’ with ST slightly elevated and positive asymmetrical T wave and unique R wave in V6 with negative asymmetrical T wave. When the QRS is less than 140 ms, the T wave in V6 may be positive.5. Frontal plane: exclusive R wave in I and aVL often with a negative asymmetrical T wave, slight ST depression and usually QS in aVR with positive T wave.6. QRS axis is variable. |
AHA: American Heart Association; ESC: European Society of Cardiology; LBBB: left bundle branch block.
Briefly, LBBB is characterized by an intraventricular conduction defect, in which interventricular septum depolarization occurs from the right to the LV, electrocardiographically manifested by a Q wave in lead V1 and a broad notched or slurred R wave in lead V6. Depolarization then continues alongside the right branch, leading to right ventricle (RV)20 depolarization with r and s waves in leads V1 and V6, respectively. LV depolarization occurs slowly and only after RV depolarization (myocyte-to-myocyte conduction from RV), emerging a S wave in lead V1 and a R′ wave in V6. Thus, the activation of the septum and LV-free wall occurs slower than in normal conduction circumstances.3
Left bundle branch block-induced dilated cardiomyopathyPathophysiologyAs previously mentioned, the relationship between LBBB and DCM is well known, particularly the traditional concept of LBBB development as a consequence of DCM progression. However, the excellent response to CRT reported in many patients with LBBB and concomitant DCM has contributed to the recognition of LBBB-iDCM as a distinct entity. Accordingly, LBBB may appear in the natural course of DCM, and present as a marker of disease severity, or it may play a causative role in LV dysfunction development. The latter corresponds to the increasingly recognized LBBB-iDCM entity, also known as dessynchronopathy.
In 2016, a scientific statement from the American Heart Association (AHA) described and presented diagnostic and management approaches for LBBB-iDCM as a specific pathological entity.21 However, ESC guidelines, published in the same year, did not contemplate this entity.22
Left bundle branch block leads to a dyssynchronous activation and contraction of the LV. The interventricular septum contracts earlier than the lateral wall, which is fully relaxed, displacing the blood in the direction of the lateral wall, which is stretched due to an abnormally high preload status. According to the Frank–Starling mechanism, activation of the lateral wall leads to its contraction, pushing blood back into the septum, which is stretched and moves towards RV, absorbing the energy coming from the lateral wall (wasted energy).23 Ultimately, contraction of the lateral wall leads to blood ejection from the LV to the systemic circulation. However, the loss of a major contribution of the septum to LV contraction causes a high afterload status, contributing to the adverse cardiac remodeling phenomenon (increased LV volume, and decreased LVEF) observed in patients with HF and LBBB.24 As a consequence of the remodeling, LBBB typically leads to lateral wall hypertrophy, as well as thinning of the septum.25 The mechanical dyssynchrony induced by LBBB can ultimately lead to LV hypertrophy, dilatation, and systolic dysfunction.
Several examples of iatrogenic dyssynchrony resemble the described mechanism. These models include LBBB development secondarily to procedural interventions (surgical or transcatheter aortic valve replacement26,27; surgical septal myectomy28) and chronic right ventricle pacing. However, these examples may not completely mimic the behavior of LBBB-iDCM.29
Not all patients with LBBB develop DCM, raising an emerging question about the existence of certain characteristics that might determine this evolution in some patients and not in others. This highlights the need for longitudinal studies enrolling patients with isolated and idiopathic LBBB without structural or functional dysfunction, to evaluate and characterize this progression to DCM, and whether it can be prevented in clinical practice, focusing on the search for possible predisposition/predicting factors.
Considering all this information, recent evidence points to the possible need for a close follow-up of LBBB patients with preserved LVEF.24 The optimal manner for stratification and surveillance (best technique and periodicity) of these “asymptomatic” LBBB carriers has not yet been established.
Diagnostic proposalsIn recent years, some studies have proposed strict criteria to help identifying LBBB-iDCM (Table 2). Nevertheless, there are no currently established recommendations on how to achieve the diagnosis. The first study introducing the LBBB-iDCM concept was published by Blanc et al.5 in 2005, showing a complete recovery of LV function one year after CRT implantation in 5 out of the 29 enrolled patients (17%). Similar results were obtained by Castellant et al.6 in 2009 and Serdoz et al.30 in 2011. In 2013, Vaillant et al.7 proposed a set of criteria to specifically identify LBBB-iDCM. Their group retrospectively screened 375 patients from a single center between 2007 and 2011 and included six patients (1.6%) that met all their proposed criteria for LBBB-iDCM diagnosis: history of typical LBBB for more than 5 years; preserved LVEF (≥50%) at the time of LBBB diagnosis; decrease in LVEF for values <40% and progressive development of HF symptoms during the follow-up; mechanical dessynchronopathy of LV; cardiomyopathy of unknown etiology; hyper-response to CRT (defined as an LVEF increase to ≥45% and a decrease in New York Heart Association (NYHA) classification to I within one year after CRT implantation). After CRT implantation, all six patients presented normalization of LV function and dimensions, resolution of mechanical dyssynchrony and improvement in HF symptoms. Despite the obvious limitations concerning the retrospective nature of the study and small number of patients, this work supported the hypothesis of a progressive development of DCM in patients with primary LBBB and the excellent therapeutic response to CRT in these cases.7 Wang et al. published two retrospective studies, one in 2016 and another in 2018, entitled of NEOLITH9 and NEOLITH II,10 respectively. Both studies demonstrated that LVEF did not significantly improve in patients with new onset LBBB-associated DCM after three months of GDMT.9,10 NEOLITH II study emphasized that earlier CRT implantation (before the three-month period of GDMT) was associated with significantly higher rates of LV recovery.10 These results were supported by Sze et al.8 who found a smaller improvement in LVEF when using GDMT in patients with DCM and LBBB, when compared with patients presenting other QRS morphologies.
Studies that contributed to the definition of the LBBB-iDCM.
| Author | Year | Study design | Number of patients | Mean age (years) | Inclusion criteria | Main findings (frequencies; response to therapies) | Proposed criteria for LBBB-iDCM diagnosis |
|---|---|---|---|---|---|---|---|
| Blanc et al.5 | 2005 | Prospective observational | 29 | 70 (±7.7) | Nonischemic DCM; sinus rhythm; LBBB; severe HF | LV function normalization (EF >50%) in 5 of 29 patients (17%) after 1 year of CRT suggesting LBBB as a reversible cause of DCM | – |
| Castellant et al.6 | 2009 | Prospective | 51 | 72.5 (±7.5) | Nonischemic DCM; sinus rhythm; permanent LBBB; severe HF | Functional recovery (NYAH I or II) and LV function normalization (EF >50%) in 11 of 51 patients (21.5%) after a minimum of 6 months of CRT | – |
| Serdoz et al.30 | 2011 | Prospective | 75 | 64 (±9) | Severe HF caused by ischemic or nonischemic DCM | LV function normalization in 13 of 75 patients after 1 year of CRT | – |
| Vaillant et al.7 | 2013 | Retrospective cohort | 375 | 50.5 | All patients between 2007 and 2010 meeting the diagnostic criteria, candidates for CRT | The entity was identified in 6 of 375 patients (1.6%) in which occurred functional recovery, normalization of LV dimensions and resolution of mechanical desynchrony | - History of typical LBBB >5 years- LV EF preserved (≥50%) at the time of LBBB diagnosis- Decreased in EF for <40% and progressive HF NYAH class II to IV during the follow-up- Mechanical dessynchronopathy of left ventricle- Cardiomyopathy with unknown etiology- Hyper-response to CRT defined as EF ≥45% and decreased NYAH class to I, after 1 year of CRT |
| Wang et al.9 | 2016 | Retrospective cohort | 102 | – | Idiopathic nonischemic cardiomyopathy; LV EF ≤35%; LBBB or narrow QRS morphology | LV EF did not significantly improve, in patients with new onset LBBB-associated nonischemic DCM, after three months of GDMT | – |
| Wang et al.10 | 2018 | Retrospective cohort | 123 | – | Idiopathic nonischemic cardiomyopathy; LV EF ≤35%; LBBB | Earlier CRT implementation (before three months of medical therapy) was associated with more significant cardiac recovery. Delayed CRT may miss a critical period to end and reverse progressive myocardial damage | – |
| Sze et al.8 | 2018 | Retrospective cohort | 659 | 59 | LV EF ≤35% on baseline echocardiogram who had a follow-up 3–6 months later | Less LV EF improvement in patients with DCM and LBBB, compared with other QRS morphologies, using GDMT.Earlier CRT is probably more suitable for patients with LBBB | – |
| Sanna et al.31 | 2021 | Retrospective cohort | 242 | 53 (±13.5) | Age >18 years; nonischemic or nonvalvular DCM; LBBB | Identification of LBBB-induced DCM in 2 of 30 patients (6.67%) with comprehensive dataset including CMR and genetic testing | The same as those described by Sanna et al., 202029:(I) ECG:- True LBBB (wide QRS (>140 ms) with notching in lateral leads and QS or rS in V2-V3- Pseudonecrosis exclusion(II) Medical history:- Exclusion of DCM family history- Exclusion of other possible causes for ventricular disfunction: coronary artery disease, toxic substances, thyroid disorders, arrhythmias, inflammatory diseases, or genetic mutations(III) Echocardiogram:- Non-severe LV dilatation and diastolic dysfunction- Normal wall thickness- Septal flash and apical rocking- Marked desynchrony- Mild left atrial dilatation- Mild functional mitral regurgitation- Normal RV function;(IV) Cardiac magnetic resonance:- Absence of late gadolinium enhancement exhibiting the absence of significant scar or fibrosis |
CMR: cardiac magnetic resonance; CRT: cardiac resynchronization therapy; DCM: dilated cardiomyopathy; ECG: electrocardiogram; EF: ejection fraction; GDMT: guideline-directed medical therapy; HF: heart failure; LBBB: left bundle branch block; LV: left ventricular; RV: right ventricle.
In 2020, Sanna et al.29 described several red flags, based on clinical observations, in patients not meeting criteria for a primary DCM diagnosis, aiming to contribute to the identification of LBBB-iDCM. These red flags comprised: (I) ECG displaying a true LBBB (wide QRS: >140 ms; notching in lateral leads; QS or rS in V2–V3; pseudonecrosis exclusion); (II) exclusion of DCM family history and other possible causes for LV dysfunction; (III) echocardiographic documentation of non-severe LV dilatation and diastolic dysfunction, normal wall thickness, septal flash and apical rocking revealing marked desynchrony, and normal RV function; (IV) absence of late gadolinium enhancement (LGE) in cardiac magnetic resonance (CMR). This study proposed an approach algorithm for patients presenting with LV dysfunction and known or newly detected LBBB. They suggest exclusion of CAD as the first step for diagnosis. Once the ischemic etiology is ruled out, the remaining above-mentioned criteria must be fulfilled for a possible LBBB-iDCM diagnosis.29
More recently, Sanna et al.31 applied the previously proposed diagnostic criteria29 to a retrospective cohort (CLIMB registry), in order to estimate the LBBB-iDCM prevalence. From the 30 enrolled patients presenting a comprehensive study, including CMR and genetic testing, 2 (6.7%) fulfilled the diagnostic criteria for LBBB-iDCM. The latter studies contributed to the definition of LBBB-iDCM as a distinct entity in patients diagnosed with DCM and LBBB. The diagnostic criteria proposed by Vaillant et al.7 are mainly based on echocardiographic characteristics, while those proposed by Sanna et al.29,31 are more stringent, as they include genetic and CMR data.
Finally, it is worth noting that there are reported cases of spontaneous resolution of LBBB leading to reverse LV remodeling,32 and that 25% of patients with LBBB and HF may respond favorably to GDMT.33
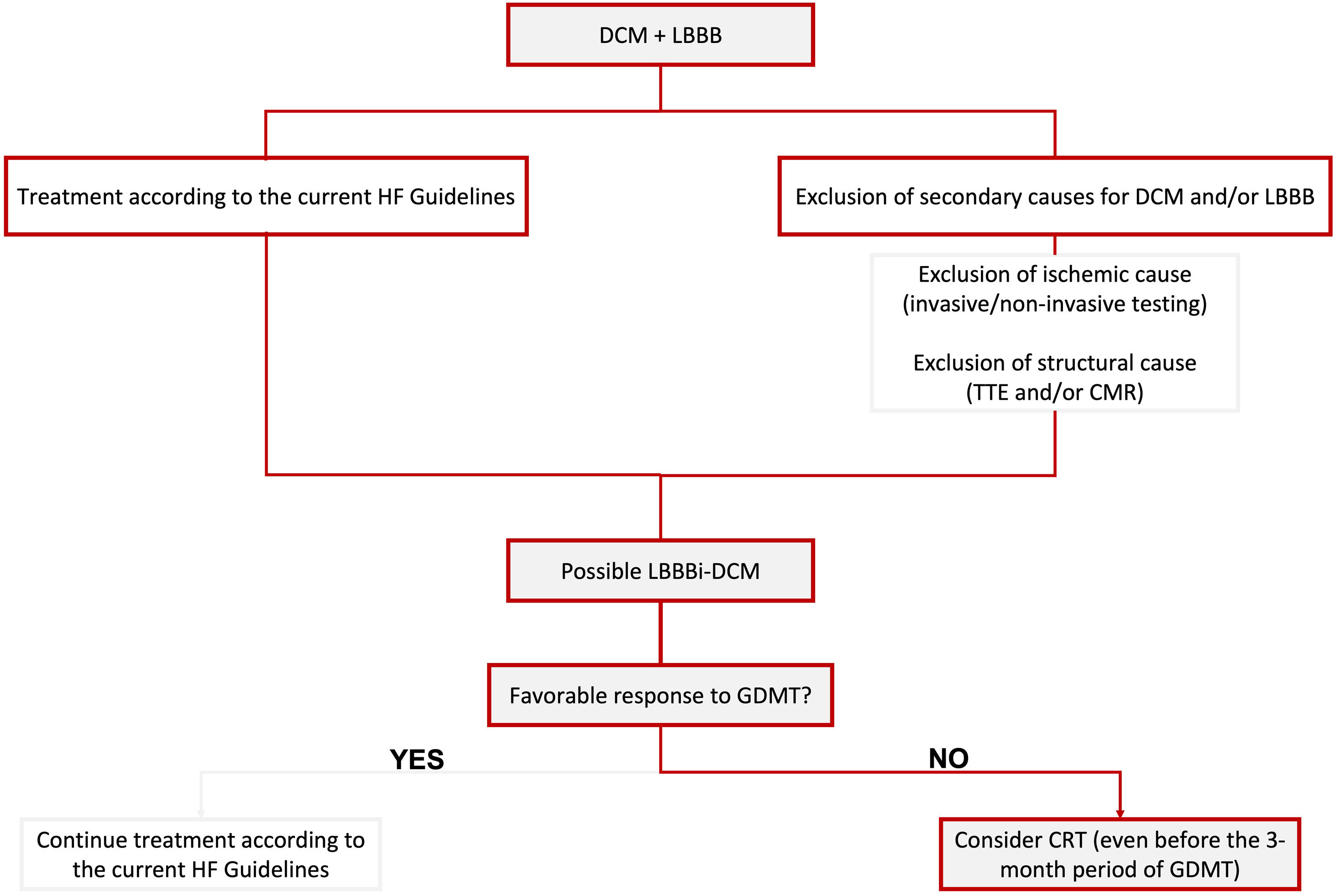
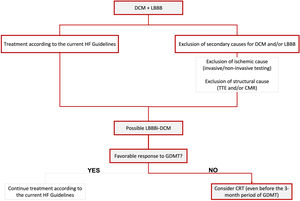
In Figure 1, we propose a simplified approach to the diagnosis of possible LBBB-iDCM, highlighting the need for exclusion of secondary causes for DCM and/or LBBB, including ischemic and structural etiologies. Concomitantly, patients should start HF treatment according to the current guideline's recommendations for HF with reduced EF (HFrEF) (≤40%).
Proposed simplified algorithm for diagnosis and treatment of LBBBi-DCM. CMR: cardiac magnetic resonance; CRT: cardiac resynchronization therapy; DCM: dilated cardiomyopathy; GDMT: guideline-directed medical therapy; HF: heart failure; LBBB: left bundle branch block; LBBBi-DCM: LBBB-induced dilated cardiomyopathy; TTE: transthoracic echocardiogram.
According to the 2022 AHA recommendations for HF management,34 first-line therapy in patients with HF with reduced EF (HFrEF) (≤40%) involves GDMT. These include the famous four pharmacological classes, namely angiotensin receptor-neprilysin inhibitor or angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, beta-blocker, mineralocorticoid receptor antagonist and sodium-glucose cotransporter 2 inhibitor. CRT is indicated in patients with persistent HFrEF (≤35%), NYHA II-IV, QRS ≥150 ms and LBBB, after three months of optimized GDMT.34 Similar recommendations were established by the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic HF.35
Several studies have shown an excellent response to CRT in patients with LBBB and DCM, as shown by LV reverse remodeling, normalization in QRS duration and improvement in LVEF.5–10,30 Strengthening this concept, a meta-analysis published in 2019 by Albakri3 demonstrated that patients with LBBB and DCM who were submitted to an early CRT strategy experienced significantly higher improvements in LVEF, when compared to the ones without LBBB.3 The overall evidence points to a worse prognosis in DCM patients who develop/present concomitant LBBB, as well as to a sub-optimal and disappointing response to GDMT in these patients, as mentioned above. On the other hand, evidence also points toward a better response to CRT in DCM patients when LBBB is present. Together, the existing data emphasize the critical role of CRT as an effective treatment for DCM patients with LBBB. They also raise awareness of the emerging question of whether this specific population should undergo an early CRT implantation approach with a view to improving their prognosis.
Another intriguing issue concerns the absence of a perfect correlation between the pre-CRT QRS duration and the QRS interval reduction after CRT with CRT response and LVEF improvement.36 In this context, some imaging-based indices have been proposed to assess the abnormal contraction pattern in LBBB patients, aiming to achieve better CRT responses. Several echocardiographic signs of dyssynchrony have been described, including septal flash, apical rocking, shorter filling and ejection time intervals and longer isovolumetric time intervals. However, to date, these echocardiographic indices have also been imperfect predictors of CRT response in LBBB patients.37–39
Although conventional biventricular pacing (BiVP) used for CRT is a well-established treatment for DCM patients with LBBB, several studies have also revealed a non-negligent risk of unsuccessful or complicated coronary venous system lead placement, as well as the existence of a significant proportion of CRT nonresponders. To overcome these issues, conduction system pacing has been proposed as an alternative to BiVP-CRT.40 In this context, in recent years promising and exciting data have arisen regarding new alternative resynchronization techniques, namely His bundle pacing (HBP)41,42 and LBB area pacing (LBBP).43,44 HBP is considered the most physiological pacing strategy, and some works have shown similar benefits to those obtained with BiVP.45,46 Nonetheless, HBP presents several challenges regarding lead fixation, and the risk of loss of capture.47 Therefore, LBBP has recently emerged as the stronger alternative to BiVP-CRT, and increasingly evidence has even suggested better performance and outcomes in patients with DCM and LBBB submitted to LBBP implantation rather than BiVP-CRT.40,44,48,49 Recent studies have shown greater LVEF improvement,49 lower arrhythmic risk,48 and reduced composite endpoint of time to death or heart failure hospitalization,44 when comparing LBBP to BiVP. Combining these results with both relatively short learning curves and short procedure times,47 growing evidence seems to support LBBP as an excellent technique for CRT. However, the best resynchronization method for LBBB-iDCM is still unknown. Further studies on the topic are already ongoing to better clarify the issue.
Despite growing evidence pointing out CRT as the cornerstone of LBBB-iDCM treatment, some challenging questions regarding medical therapy in this population are still unsolved. The unfavorable response to HF pharmacological therapy in these subjects may reflect the lack of a targeted approach to the pathophysiologic mechanisms underlying LBBB-iDCM development. The same justification supports the documentation of an excellent response to CRT in these patients. Need for HF medical therapy maintenance in patients presenting a complete LV function recovery after CRT implantation remains unclear. Some studies have tried to address the question but with contradictory results. TRED-HF50 was the first randomized trial evaluating the safety and feasibility of HF medical therapy withdrawal in fully recovered DCM patients. Around 40% of the enrolled patients presented recurrence within the 6-month follow-up period after medical therapy suspension. STOP-CRT51 was a pilot trial aiming to evaluate a CRT-only strategy in patients with recovered LVEF after CRT. Around 8% of patients presented recurrence within the two-year follow-up period. The major discrepancy between relapse rates in TRED-HF and STOP-CRT is justifiable, among other factors, by differences in patient selection. Differently from TRED-HF, patients enrolled in STOP-CRT presented recovered LVEF due to cardiac resynchronization and not due to medical therapy. Once again, these results support the critical role of CRT in the treatment of LBBB-iDCM patients, in whom desynchrony is the main or even sole etiology of DCM. Acting directly on the pathophysiologic substrate of the disease through CRT may be the key to reverse DCM, suggesting that medical therapy may present a minor/secondary role in these cases. However, these results should obviously be interpreted with caution and are currently hypothesis generating. Future studies should be designed to address this question properly.
Lastly, another intriguing issue relates to the need for defibrillator implantation in these patients for sudden cardiac death (SCD) primary prevention. The evidence supporting recommendations for implantable cardioverter-defibrillator (ICD) in HFrEF patients35 is much more robust for ischemic rather than nonischemic cardiomyopathy.52 In LBBB-iDCM, as previously mentioned, the reversal of the conduction abnormality may translate into a complete LVEF recovery. In this context, a recent prospective single-center observational study53 explored the feasibility of implanting LBBP with or without a defibrillator in LBBB-iDCM patients with LVEF ≤35% according to LGE-CMR risk stratification. The extent of LGE is a known predictor of SCD risk in DCM.54 The underlying minimal mortality benefit provided by prophylactic ICD in LBBB-iDCM, as well as their typical excellent response to CRT led the authors to hypothesize that LGE-CMR could be used for stratification in this subgroup of patients. Their results point to LGE-CMR extent as a possible future feature to aid in the risk stratification of LBBB-iDCM. Theoretically, patients with a low LGE burden could be excluded from ICD, while the ones with higher LGE burden would benefit from ICD. More studies and randomized trials are needed to help clarify these findings, and further clarify the need for ICD in LBBB-iDCM.
In Figure 1, we briefly focus on treatment approaches, highlighting the need to consider CRT as the cornerstone of treatment, mainly when a favorable response to GDMT is not achieved (which should correspond to most cases, according to the current literature). We also emphasize the need to contemplate CRT before the three-month period of optimal GDMT when a sub-optimal response is being observed.
ConclusionsLeft bundle branch block is a common finding in DCM patients, but its role as a cause or consequence is not yet well established. LBBB-iDCM has emerged as a distinct pathological entity mainly due to its excellent response to CRT with LV reverse remodeling and complete LVEF recovery, highlighting CRT as the apparent cornerstone of treatment. Additionally, growing evidence has shown that patients with LBBB and DCM present poorer outcomes after three months of GDMT. This raises the hypothesis of early CRT implantation as a critical step in the treatment of these patients.
Despite strong evidence highlighting the existence of LBBB-iDCM, there are no current universal criteria for its diagnosis. More prospective studies are needed to better understand and characterize this entity. It is also important to better clarify CRT role in the treatment of this population, particularly in terms of appropriate timing for implantation, and selection of the most adequate resynchronization technique, as it can positively change the outcomes and, consequently, change current recommendations in patients with LBBB-iDCM.
Conflicts of interestThe authors have no conflicts of interest to declare.