Sleep apnea syndrome (SAS) is a common respiratory disorder that is particularly prevalent in patients with cardiovascular disease. Diagnosis is based on polysomnography. In patients with cardiac implantable electronic devices (CIEDs), the prevalence of SAS may reach 60%. The aim of this study was to assess the value of CIEDs in screening for SAS.
MethodsThis prospective study included patients with CIEDs with an algorithm for sleep apnea. The frequency response function was activated and simplified polysomnography was performed. The device’s data were collected on the day of the polygraph.
ResultsThe sample included 29 patients, with a mean age of 76.1 years, 71.4% male. The prevalence of SAS was 77%. For SAS, the agreement between polysomnography and the device was kappa=0.54 (p=0.001, 95% CI 0.28-0.81) (moderate agreement); for moderate to severe SAS, the agreement was kappa=0.73 (p<0.001, 95% CI 0.49-0.976) (substantial agreement). The following values were obtained for severe SAS: sensitivity 60%, specificity 100%, positive predictive value (PPV) 100%, negative predictive value (NPV) 60%, and diagnostic accuracy 75%; for moderate to severe SAS: sensitivity 90%, specificity 83%, PPV 90%, NPV 87.5%, and diagnostic accuracy 87.5%.
ConclusionSAS is highly prevalent in patients with CIEDs. The values obtained through these devices have a strong positive correlation with the apnea-hypopnea index, which makes them a good tool for the screening of severe SAS.
A Síndrome da Apneia do Sono (SAS) é uma doença respiratória prevalente, com expressão marcada na população com doença cardiovascular. O diagnóstico é baseado na polissonografia. Nos doentes com dispositivos eletrónicos cardíacos (DEC), a prevalência de SAS pode atingir 60%. O objetivo deste estudo foi avaliar o valor dos DEC no rastreio de SAS.
MétodosEstudo prospetivo que incluiu doentes com DEC com algoritmo de apneia do sono. Foi ativada a função de resposta em frequência e realizada a poligrafia simplificada. Foram recolhidos os dados do dispositivo no dia em que foi realizada a poligrafia.
ResultadosA amostra incluiu 29 doentes, com uma idade média de 76,1 anos, 71,4% do género masculino. A prevalência de SAS foi de 77%. Para SAS grave a concordância entre a polissonografia e o pacemaker foi de Kappa=0,54 (p=0,001), IC 95% (0,28, 0,81) (concordância moderada); para SAS moderada a grave, a concordância foi de Kappa = 0,73 (p<0,001), IC 95% (0,49, 0,976) (concordância substancial). Para SAS grave obtiveram-se: sensibilidade 60%, especificidade de 100%, valor preditivo positivo de 100%, valor preditivo negativo de 60% e acurácia diagnóstica de 75%; para SAS moderada a grave: sensibilidade de 90%, especificidade de 83%, valores preditivo positivo de 90% e negativo de 87,5%, com acurácia diagnóstica de 87,5%.
ConclusãoA SAS é altamente prevalente nos portadores de DEC. Os valores obtidos através destes dispositivos apresentam uma correlação positiva forte com o índice de Apneia-Hipopneia, o que faz deles uma boa ferramenta de rastreio de SAS grave.
Sleep apnea syndrome (SAS) is a common respiratory disorder that is caused by intermittent collapse of the airway during sleep, interrupting (apnea) or decreasing (hypopnea) normal respiration. The number of apnea or hypopnea events/hour is used to calculate the apnea/hypopnea index (AHI) and, when associated with respiratory-effort related arousals, the respiratory disturbance index.1
The cause of sleep apnea may be obstructive (more common) or central, the latter being diagnosed when the number of respiratory events of central cause is more than 50% of the total number of events detected on polysomnography (PSG).1
Studies on SAS have shown that the prevalence of AHI >5 events/hour and AHI >15 can reach 20% and over 15%, respectively, in the adult population.2 In individuals aged over 70 years, prevalences of obstructive sleep apnea as high as 20% have been reported,3 while in patients with cardiovascular disease, the prevalence of sleep apnea can range between 47% and 83%, depending on the specific disorder surveyed.4 In patients with cardiac implantable electronic devices (CIEDs), the prevalence of SAS may reach nearly 60%.5
The aim of this study was to assess the value of CIEDs in screening for SAS.
MethodsThe sample included 29 patients with CIEDs (pacemakers, implantable cardioverter-defibrillators [ICDs] and cardiac resynchronization [CRT] devices) implanted between January 2013 and January 2015 that included a sleep apnea/hypopnea monitoring algorithm (Kora™ 100 DR and Reply™ 200 DR, LivaNova; Vitalio™, Incepta™ CRT-D, Incepta™ ICD and INVIVE™, Boston Scientific).
The study was authorized by the hospital's ethics committee and patients’ informed consent was obtained before their inclusion in the study. Patients’ demographic, anthropometric and clinical data were collected.
The LivaNova devices are equipped with a sleep apnea monitoring (SAM) algorithm that detects apneas (defined as a period of 10-60 s between respiratory cycles) and hypopneas (defined as a reduction of 50% or more in respiratory amplitude). The SAM algorithm is able to identify individuals with severe SAS, defined as those with more than 20 events/hour (equivalent to AHI >30/hour), in accordance with the results of the DREAM study.6
The devices from Boston Scientific used in this study detect apnea and hypopnea events using ApneaScan™ and AP Scan™, which can also identify patients with severe SAS, using a cutoff of 30 events/hour (equivalent to AHI >30/hour).
Patients underwent simplified PSG with the ApneaLink Plus™ device (ResMed Corporation, Poway, CA), equipped with an effort sensor, nasal pressure cannula and oximetry, and with cardiopulmonary monitoring facilities including heart rate and peripheral oxygen saturation (SpO2). Apnea and hypopnea events were quantified and snoring events and Cheyne-Stokes breathing were identified.
The results were assessed in all cases by the same team in the pneumology department. Apnea events were defined as those in which there was no respiratory flow for 10 s or more, while hypopnea events were defined as a reduction of 30% or more in amplitude in one respiratory cycle accompanied by a fall of at least 3% in SpO2.
A diagnosis of SAS was made in the presence of AHI >5 associated with characteristic symptoms or increased cardiovascular risk,1 or of AHI >15 without symptoms.
Following observation by the pneumology team and resolution of doubtful cases by type 2 (outpatient) PSG, patients with a confirmed diagnosis of SAS were started on positive pressure therapy (continuous positive airway pressure [CPAP], bilevel positive airway pressure or adaptive servo-ventilation) in accordance with international guidelines and the specific disorder identified. They were monitored by their CIED to determine adherence to therapy.
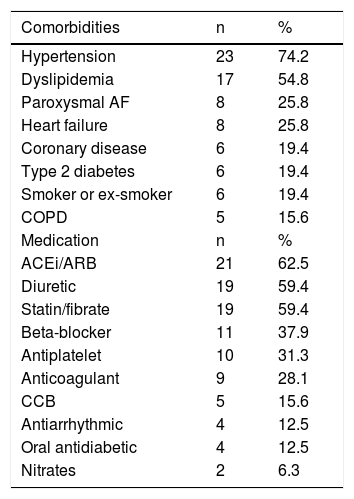
ResultsOf the 29 patients in the study, 21 were male (71.4%) and eight female (27.6%). Mean age was 76.1±9.8 years and mean body mass index (BMI) was 26.8±4.2 kg/m2. Table 1 displays the prevalence of comorbidities and medical therapy prescribed in the study population. Hypertension was the most frequent comorbidity, and 63.3% of patients had normal left ventricular systolic function on echocardiographic assessment. The CIEDs implanted were a permanent pacemaker in 23 patients (for sinus node dysfunction in 13 cases and atrioventricular conduction disturbances in the other eight, all dual-chamber devices in DDDR mode), a CRT-D in five patients, and a dual-chamber ICD in one. Fifteen of the devices were from LivaNova and 14 were from Boston Scientific.
Comorbidities and medical therapy in the study population.
| Comorbidities | n | % |
|---|---|---|
| Hypertension | 23 | 74.2 |
| Dyslipidemia | 17 | 54.8 |
| Paroxysmal AF | 8 | 25.8 |
| Heart failure | 8 | 25.8 |
| Coronary disease | 6 | 19.4 |
| Type 2 diabetes | 6 | 19.4 |
| Smoker or ex-smoker | 6 | 19.4 |
| COPD | 5 | 15.6 |
| Medication | n | % |
| ACEi/ARB | 21 | 62.5 |
| Diuretic | 19 | 59.4 |
| Statin/fibrate | 19 | 59.4 |
| Beta-blocker | 11 | 37.9 |
| Antiplatelet | 10 | 31.3 |
| Anticoagulant | 9 | 28.1 |
| CCB | 5 | 15.6 |
| Antiarrhythmic | 4 | 12.5 |
| Oral antidiabetic | 4 | 12.5 |
| Nitrates | 2 | 6.3 |
ACEi/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; AF: atrial fibrillation; CCB: calcium channel blocker; COPD: chronic obstructive pulmonary disease.
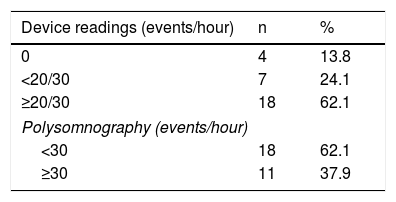
Analysis of the device readings showed that in four patients no events were recorded, seven had <20 events/hour (LivaNova) or <30 events/hour (Boston Scientific) and 18 had ≥20/30 events/hour (Table 2).
On the PSG study, 18 patients (62%) had AHI <15/hour, 20 (68.9%) had AHI 15-30/hour and 11 (37.9%) had AHI >30/hour.
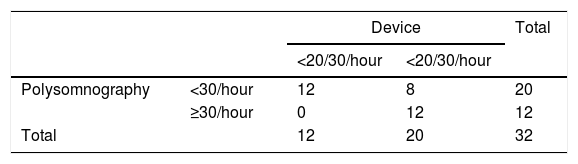
Joint analysis of data from the devices and from the PSG study (Table 3) shows that the 11 patients who had AHI ≥30/hour all had ≥20/30/hour recorded by their CIEDs. However, in seven cases the PSG result was <30/hour, while the device reading was ≥20/30/hour. For SAS, the agreement between polysomnography and the device was kappa=0.54 (p=0.001, 95% confidence interval [CI] 0.28-0.81) (moderate agreement).
Assessment of the agreement between the two methods shows that of the 20 patients with AHI >15/hour on PSG (moderate to severe SAS), 18 had ≥20/30 events/hour recorded by their device. Two patients with AHI <15/hour on PSG also had ≥20/30 events/hour recorded by their device. Agreement between PSG and the device was kappa=0.73 (p<0.001, 95% CI 0.49-0.976) (substantial agreement).
When device values of >20/30/hour were correlated with PSG >30/hour, the following values were obtained: sensitivity 60%, specificity 100%, positive predictive value (PPV) 100%, negative predictive value (NPV) 60%, and diagnostic accuracy 75%; for PSG >15/hour: sensitivity 90%, specificity 83%, PPV 90%, NPV 87.5%, and diagnostic accuracy 87.5%.
Mean BMI was not significantly different between individuals with PSG <30/hour and ≥30/hour (26.75 vs. 26.98, p=0.886).
DiscussionThe international guidelines recommend full PSG as the gold standard for diagnosing patients with suspected SAS.1 Home testing with portable monitors can also be used in individuals with a high pretest likelihood of moderate to severe SAS without other sleep disorders or other major comorbidities.7 Full PSG records the number of apnea or hypopnea events, from which the AHI or respiratory disturbance index can be calculated, while simplified PSG only expresses its results in terms of the AHI. Although the latter, when used at home, reduces the costs of hospitalizations, there is a long waiting list for this test at many centers, due to the high prevalence of sleep disorders and the fact that it cannot be performed in a primary health care context. The significant association between SAS and cardiovascular morbidity – including hypertension,8–10 acute coronary syndrome,11 supraventricular arrhythmias including atrial fibrillation12–15 as well as ventricular arrhythmias,14 coronary disease, heart failure and stroke16–20 – means that the exam should be performed promptly when symptoms suggest a diagnosis of the syndrome.
In patients with CIEDs, among whom the prevalence of SAS is significant, the detection of apnea/hypopnea events by their device can be a useful way to identify individuals who should undergo PSG.
In our study, 37.9% of patients had AHI ≥30 events/hour and 68.9% had >15/hour, reflecting a high prevalence of SAS in this population, which is in agreement with findings in the literature. This high prevalence may be related to the mean age of the study population (76.1±9.8 years). In a study by Defaye et al., the mean age of the sample was 73.8±19.1 years and the prevalence of SAS was 78%.6
Interestingly, the mean BMI in individuals with PSG <30/hour and those with ≥30/hour did not differ significantly, which highlights the need to pay due attention to symptoms of SAS even in individuals with lower BMI.
Agreement between device readings and PSG values for AHI >30/hour was moderate, rising to substantial for AHI >15/hour (moderate SAS), with therapeutic implications. For AHI >30/hour on PSG, the device readings had sensitivity 60%, specificity 100%, PPV 100%, NPV 60%, and diagnostic accuracy 75%; for AHI >15/hour, sensitivity was 90%, specificity 83%, PPV 90%, NPV 87.5%, and diagnostic accuracy 87.5%. These figures are in line with those in the literature6 and highlight the role of CIEDs in screening for moderate to severe SAS, which has significant implications for diagnosis, treatment and quality of life.
The high prevalence of SAS in the population with CIEDs has two implications. Firstly, cardiologists need to be more aware of the need to screen for this syndrome and to confirm the diagnosis by PSG and, in patients with CIEDs that include detection algorithms, by analyzing event records from these devices and making this important information, which is often undervalued, available to the attending cardiologist. Secondly, the ability to monitor patients via their CIED after beginning positive pressure therapy is of considerable clinical utility for assessing patient adherence to therapy, since some studies have demonstrated levels of adherence as low as 65-80%.21
Although the data are inconclusive, some studies have proposed enhancing device programming to help reduce apnea/hypopnea events. A 2009 meta-analysis22 of studies evaluating atrial overdrive pacing for the treatment of SAS found conflicting results, some studies showing the therapy to be effective and others showing no effect.25 Garrigue et al. showed that atrial overdrive pacing at 15 bpm faster than mean nocturnal baseline heart rate reduced the AHI by 60%.23 Two mechanisms have been put forward to explain this effect. One is that overdrive pacing increases cardiac output and thereby decreases pulmonary congestion, improving breathing frequency. The other is that it counteracts nocturnal hypervagotonia by influencing cardiac sympathetic afferent neurons, thus stabilizing respiration.24 However, this study showed that individuals without signs of heart failure and without indication for antibradyarrhythmia pacing did not benefit from atrial overdrive pacing, and that those with central sleep apnea benefited more from this therapy.
CIEDs with the ability to detect apnea events thus appear to be a cost-effective alternative to diagnose and, potentially, to treat SAS.26,27
ConclusionsSAS is highly prevalent in patients with CIEDs. Several of these devices (at present from only two manufacturers) are equipped with algorithms that can record apnea/hypopnea events and can thereby identify patients who should be referred for full PSG to confirm a diagnosis of SAS. The present study has shown a significant correlation between readings from these CIEDs and the results of PSG.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ribeiro S, Bonito L, Guimarães MJ, Português J, Rodrigues B, Alves A, et al. Importância dos dispositivos eletrónicos cardíacos implantáveis no diagnóstico da Síndrome da Apneia do Sono. Rev Port Cardiol. 2019;38:451–455.